Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression
- PMID: 21712814
- PMCID: PMC3222520
- DOI: 10.1038/mt.2011.119
Systemic administration of AAV8-α-galactosidase A induces humoral tolerance in nonhuman primates despite low hepatic expression
Abstract
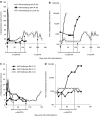
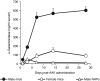
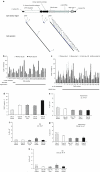
In mice, liver-restricted expression of lysosomal enzymes from adeno-associated viral serotype 8 (AAV8) vectors results in reduced antibodies to the expressed proteins. To ask whether this result might translate to patients, nonhuman primates (NHPs) were injected systemically with AAV8 encoding α-galactosidase A (α-gal). As in mice, sustained expression in monkeys attenuated antibody responses to α-gal. However, this effect was not robust, and sustained α-gal levels were 1-2 logs lower than those achieved in male mice at the same vector dose. Because our mouse studies had shown that antibody levels were directly related to expression levels, several strategies were evaluated to increase expression in monkeys. Unlike mice, expression in monkeys did not respond to androgens. Local delivery to the liver, immune suppression, a self-complementary vector and pharmacologic approaches similarly failed to increase expression. While equivalent vector copies reached mouse and primate liver and there were no apparent differences in vector form, methylation or deamination, transgene expression was limited at the mRNA level in monkeys. These results suggest that compared to mice, transcription from an AAV8 vector in monkeys can be significantly reduced. They also suggest some current limits on achieving clinically useful antibody reduction and therapeutic benefit for lysosomal storage diseases using a systemic AAV8-based approach.
Figures


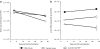
References
-
- Ge Y, Powell S, Van Roey M., and, McArthur JG. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 2001;97:3733–3737. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

