SK2 channels are neuroprotective for ischemia-induced neuronal cell death
- PMID: 21712833
- PMCID: PMC3323193
- DOI: 10.1038/jcbfm.2011.90
SK2 channels are neuroprotective for ischemia-induced neuronal cell death
Abstract
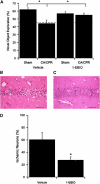
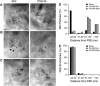
In mouse hippocampal CA1 pyramidal neurons, the activity of synaptic small-conductance Ca(2+)-activated K(+) channels type 2 (SK2 channels) provides a negative feedback on N-methyl-D-aspartate receptors (NMDARs), reestablishing Mg(2+) block that reduces Ca(2+) influx. The well-established role of NMDARs in ischemia-induced excitotoxicity led us to test the neuroprotective effect of modulating SK2 channel activity following cerebral ischemia induced by cardiac arrest and cardiopulmonary resuscitation (CA/CPR). Administration of the SK channel positive modulator, 1-ethyl-benzimidazolinone (1-EBIO), significantly reduced CA1 neuron cell death and improved CA/CPR-induced cognitive outcome. Electrophysiological recordings showed that CA/CPR-induced ischemia caused delayed and sustained reduction of synaptic SK channel activity, and immunoelectron microscopy showed that this is associated with internalization of synaptic SK2 channels, which was prevented by 1-EBIO treatment. These results suggest that increasing SK2 channel activity, or preventing ischemia-induced loss of synaptic SK2 channels, are promising and novel approaches to neuroprotection following cerebral ischemia.
Figures



References
-
- Ballanyi K. Protective role of neuronal KATP channels in brain hypoxia. J Exp Biol. 2004;207:3201–3212. - PubMed
-
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43:1369–1374. - PubMed
-
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. - PubMed
-
- Boast CA, Gerhardt SC, Pastor G, Lehmann J, Etienne PE, Liebman JM. The N-methyl-D-aspartate antagonists CGS 19755 and CPP reduce ischemic brain damage in gerbils. Brain Res. 1988;442:345–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

