The naturally processed CD95L elicits a c-yes/calcium/PI3K-driven cell migration pathway
- PMID: 21713032
- PMCID: PMC3119658
- DOI: 10.1371/journal.pbio.1001090
The naturally processed CD95L elicits a c-yes/calcium/PI3K-driven cell migration pathway
Erratum in
-
Correction: The Naturally Processed CD95L Elicits a c-Yes/Calcium/PI3K-Driven Cell Migration Pathway.PLoS Biol. 2019 Oct 8;17(10):e3000521. doi: 10.1371/journal.pbio.3000521. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31593568 Free PMC article.
-
Correction: The Naturally Processed CD95L Elicits a c-Yes/Calcium/PI3K-Driven Cell Migration Pathway.PLoS Biol. 2023 Feb 23;21(2):e3002027. doi: 10.1371/journal.pbio.3002027. eCollection 2023 Feb. PLoS Biol. 2023. PMID: 36821824 Free PMC article.
Abstract
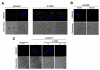
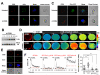
Patients affected by chronic inflammatory disorders display high amounts of soluble CD95L. This homotrimeric ligand arises from the cleavage by metalloproteases of its membrane-bound counterpart, a strong apoptotic inducer. In contrast, the naturally processed CD95L is viewed as an apoptotic antagonist competing with its membrane counterpart for binding to CD95. Recent reports pinpointed that activation of CD95 may attract myeloid and tumoral cells, which display resistance to the CD95-mediated apoptotic signal. However, all these studies were performed using chimeric CD95Ls (oligomerized forms), which behave as the membrane-bound ligand and not as the naturally processed CD95L. Herein, we examine the biological effects of the metalloprotease-cleaved CD95L on CD95-sensitive activated T-lymphocytes. We demonstrate that cleaved CD95L (cl-CD95L), found increased in sera of systemic lupus erythematosus (SLE) patients as compared to that of healthy individuals, promotes the formation of migrating pseudopods at the leading edge of which the death receptor CD95 is capped (confocal microscopy). Using different migration assays (wound healing/Boyden Chamber/endothelial transmigration), we uncover that cl-CD95L promotes cell migration through a c-yes/Ca²⁺/PI3K-driven signaling pathway, which relies on the formation of a CD95-containing complex designated the MISC for Motility-Inducing Signaling Complex. These findings revisit the role of the metalloprotease-cleaved CD95L and emphasize that the increase in cl-CD95L observed in patients affected by chronic inflammatory disorders may fuel the local or systemic tissue damage by promoting tissue-filtration of immune cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Trauth B. C, Klas C, Peters A. M, Matzku S, Moller P, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–305. - PubMed
-
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. - PubMed
-
- Siegel R. M, Frederiksen J. K, Zacharias D. A, Chan F. K, Johnson M, et al. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. - PubMed
-
- Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, et al. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276:23954–23961. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

