Nitric Oxide Synthase Inhibitor Improves De Novo and Long-Term l-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats
- PMID: 21713068
- PMCID: PMC3114204
- DOI: 10.3389/fnsys.2011.00040
Nitric Oxide Synthase Inhibitor Improves De Novo and Long-Term l-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats
Abstract
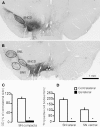
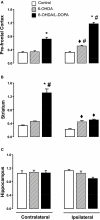
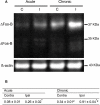
Inhibitors of neuronal and endothelial nitric oxide synthase decrease l-3,4-dihidroxifenilalanine (l-DOPA)-induced dyskinesias in rodents. The mechanism of nitric oxide inhibitor action is unknown. The aims of the present study were to investigate the decrease of l-DOPA-induced abnormal involuntary movements (AIMs) in 6-hydroxydopamine (6-OHDA)-lesioned rats by nitric oxide inhibitors following either acute or chronic treatment. The primary findings of this study were that NG-nitro-l-Arginine, an inhibitor of endothelial and neuronal nitric oxide synthase, attenuated AIMs induced by chronic and acute l-DOPA. In contrast, rotational behavior was attenuated only after chronic l-DOPA. The 6-OHDA lesion and the l-DOPA treatment induced a bilateral increase (1.5 times) in the neuronal nitric oxide synthase (nNOS) protein and nNOS mRNA in the striatum and in the frontal cortex. There was a parallel increase, bilaterally, of the FosB/ΔFosB, primarily in the ipsilateral striatum. The exception was in the contralateral striatum and the ipsilateral frontal cortex, where chronic l-DOPA treatment induced an increase of approximately 10 times the nNOS mRNA. Our results provided further evidence of an anti-dyskinetic effect of NOS inhibitor. The effect appeared under l-DOPA acute and chronic treatment. The l-DOPA treatment also revealed an over-expression of the neuronal NOS in the frontal cortex and striatum. Our results corroborated findings that l-DOPA-induced rotation differs between acute and chronic treatment. The effect of the NOS inhibitor conceivably relied on the l-DOPA structural modifications in the Parkinsonian brain. Taken together, these data provided a rationale for further evaluation of NOS inhibitors in the treatment of l-DOPA-induced dyskinesia.
Keywords: FosB/ΔFosB; Parkinson's disease; abnormal involuntary movements; l-DOPA-induced dyskinesia; nitric oxide; nitric oxide synthase; nitric oxide synthase inhibitors; striatum.
Figures
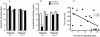



References
-
- Abreu-González P., González-Hernández T., Afonso-Oramas D., Cruz-Muros I., Barroso-Chinea P., González M. C. (2006). Tetrahydrobiopterin stimulates l-DOPA release from striatal tissue. Eur. J. Pharmacol. 541, 33–37 - PubMed
-
- Andersson M., Hilbertson A., Cenci M. A. (1999). Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol. Dis. 6, 461–474 - PubMed
-
- Aquilano K., Baldelli S., Rotilio G., Ciriolo MR. (2008). Role of nitric oxide synthases in Parkinson's disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem. Res. 33, 2416–2426 - PubMed
-
- Barone P. (2010). Neurotransmission in Parkinson's disease: beyond dopamine. Eur. J. Neurol. 17, 364–376 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

