Understanding the molecular diversity of GABAergic synapses
- PMID: 21713106
- PMCID: PMC3112311
- DOI: 10.3389/fncel.2011.00004
Understanding the molecular diversity of GABAergic synapses
Abstract
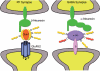
GABAergic synapses exhibit a high degree of subcellular and molecular specialization, which contrasts with their apparent simplicity in ultrastructural appearance. Indeed, when observed in the electron microscope, GABAergic synapses fit in the symmetric, or Gray's type II category, being characterized by a relatively simple postsynaptic specialization. The inhibitory postsynaptic density cannot be readily isolated, and progress in understanding its molecular composition has lagged behind that of excitatory synapses. However, recent studies have brought significant progress in the identification of new synaptic proteins, revealing an unexpected complexity in the molecular machinery that regulates GABAergic synaptogenesis. In this article, we provide an overview of the molecular diversity of GABAergic synapses, and we consider how synapse specificity may be encoded by selective trans-synaptic interactions between pre- and postsynaptic adhesion molecules and secreted factors that reside in the synaptic cleft. We also discuss the importance of developing cataloguing tools that could be used to decipher the molecular diversity of synapses and to predict alterations of inhibitory transmission in the course of neurological diseases.
Keywords: GABAA receptor; GABAergic synapse; dystroglycan; gephyrin; neurexin; neuroligin; synapse specificity.
Figures