An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons
- PMID: 21713123
- PMCID: PMC3113108
- DOI: 10.3389/fnsys.2011.00032
An Improved BAC Transgenic Fluorescent Reporter Line for Sensitive and Specific Identification of Striatonigral Medium Spiny Neurons
Abstract
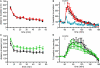
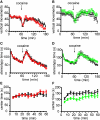
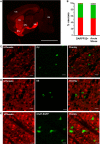
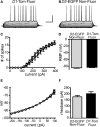
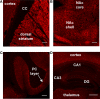
The development of BAC transgenic mice expressing promoter-specific fluorescent reporter proteins has been a great asset for neuroscience by enabling detection of neuronal subsets in live tissue. For the study of basal ganglia physiology, reporters driven by type 1 and 2 dopamine receptors have been particularly useful for distinguishing the two classes of striatal projection neurons - striatonigral and striatopallidal. However, emerging evidence suggests that some of the transgenic reporter lines may have suboptimal features. The ideal transgenic reporter line should (1) express a reporter with high sensitivity and specificity for detecting the cellular subset of interest and that does not otherwise alter the biology of the cells in which it is expressed, and (2) involve a genetic manipulation that does not cause any additional genetic effects other than expression of the reporter. Here we introduce a new BAC transgenic reporter line, Drd1a-tdTomato line 6, with features that approximate these ideals, offering substantial benefits over existing lines. In this study, we investigate the integrity of dopamine-sensitive behaviors and test the sensitivity and specificity of tdTomato fluorescence for identifying striatonigral projection neurons in mice. Behaviorally, hemizygous Drd1a-tdTomato line 6 mice are similar to littermate controls; while hemizygous Drd2-EGFP mice are not. In characterizing the sensitivity and specificity of line 6 mice, we find that both are high. The results of this characterization indicate that line 6 Drd1a-tdTomato+/- mice offer a useful alternative approach to identify both striatonigral and striatopallidal neurons in a single transgenic line with a high degree of accuracy.
Keywords: BAC; basal ganglia; direct pathway; medium spiny neuron; striatonigral; striatum; tdTomato; transgenic.
Figures
References
-
- Anderson K. D., Reiner A. (1991). Immunohistochemical localization of DARPP-32 in striatal projection neurons and striatal interneurons: implications for the localization of D1-like dopamine receptors on different types of striatal neurons. Brain Res. 568, 235–24310.1016/0006-8993(91)91403-N - DOI - PubMed
-
- Bertran-Gonzalez J., Bosch C., Maroteaux M., Matamales M., Herve D., Valjent E., Girault J. A. (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28, 5671–568510.1523/JNEUROSCI.1039-08.2008 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

