Protein-protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1
- PMID: 21713383
- PMCID: PMC6545597
- DOI: 10.1007/s00210-011-0646-6
Protein-protein interactions: a mechanism regulating the anti-metastatic properties of Nm23-H1
Abstract
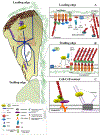
Nm23-H1, also known as NDPK-A, was the first of a class of metastasis suppressor genes to be identified. Overexpression of Nm23-H1 in metastatic cell lines (melanoma, breast carcinoma, prostate, colon, hepatocellular, and oral squamous cell carcinoma) reduced cell motility in in vitro assays and metastatic potential in xenograft models, without a significant effect on primary tumor size. The mechanism of Nm23-H1 suppression of metastasis, however, is incompletely understood. Nm23-H1 has been reported to bind proteins, including those in small G-protein complexes, transcriptional complexes, the Map kinase, the TGF-β signaling pathways and the cytoskeleton. Evidence supporting these associations is presented together with evidence of resultant biochemical and phenotypic consequences of association. Cumulatively, the data suggest that part of the anti-metastatic function of Nm23-H1 lies in pathways that it interrupts via binding and inactivation of proteins.
Figures
References
-
- Arnaud-Dabernat S, Bourbon PM, Dierich A, Le Meur M, Daniel JY (2003) Knockout mice as model systems for studying nm23/NDP kinase gene functions. Application to the nm23-M1 gene. J Bioenerg Biomembr 35(1):19–30 - PubMed
-
- Baxi MD, Vishwanatha JK (1995) Uracil DNA-glycosylase/glyceraldehyde-3-phosphate dehydrogenase is an Ap4A binding protein. Biochemistry 34(30):9700–9707 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

