Warfarin glycosylation invokes a switch from anticoagulant to anticancer activity
- PMID: 21714096
- PMCID: PMC3217245
- DOI: 10.1002/cmdc.201100178
Warfarin glycosylation invokes a switch from anticoagulant to anticancer activity
Figures


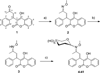

Similar articles
-
The characterization of potent novel warfarin analogs.Thromb Res. 1997 Oct 15;88(2):127-36. doi: 10.1016/s0049-3848(97)00224-7. Thromb Res. 1997. PMID: 9361366
-
Synthesis and structure-activity relationships of novel warfarin derivatives.Bioorg Med Chem. 2007 Mar 15;15(6):2414-20. doi: 10.1016/j.bmc.2007.01.014. Epub 2007 Jan 17. Bioorg Med Chem. 2007. PMID: 17275317
-
[Vitamin K epoxide reductase: Fresh blood for oral anticoagulant therapies].Rev Med Interne. 2006 Dec;27(12):979-82. doi: 10.1016/j.revmed.2006.09.004. Epub 2006 Oct 11. Rev Med Interne. 2006. PMID: 17070618 Review. French.
-
Species comparison of vitamin K1 2,3-epoxide reductase activity in vitro: kinetics and warfarin inhibition.Toxicology. 2003 Aug 1;189(3):191-8. doi: 10.1016/s0300-483x(03)00133-1. Toxicology. 2003. PMID: 12832152
-
Warfarin and the vitamin K-dependent gamma-carboxylation system.Trends Mol Med. 2004 Jul;10(7):299-302. doi: 10.1016/j.molmed.2004.05.003. Trends Mol Med. 2004. PMID: 15242675 Review.
Cited by
-
Natural product disaccharide engineering through tandem glycosyltransferase catalysis reversibility and neoglycosylation.Org Lett. 2012 Oct 5;14(19):5086-9. doi: 10.1021/ol3023374. Epub 2012 Sep 17. Org Lett. 2012. PMID: 22984807 Free PMC article.
-
Synthesis and antibacterial activity of doxycycline neoglycosides.J Nat Prod. 2013 Sep 27;76(9):1627-36. doi: 10.1021/np4003096. Epub 2013 Aug 29. J Nat Prod. 2013. PMID: 23987662 Free PMC article.
-
Dual targeting of the Warburg effect with a glucose-conjugated lactate dehydrogenase inhibitor.Chembiochem. 2013 Nov 25;14(17):2263-7. doi: 10.1002/cbic.201300562. Epub 2013 Oct 31. Chembiochem. 2013. PMID: 24174263 Free PMC article.
-
Direct synthesis of β-N-glycosides by the reductive glycosylation of azides with protected and native carbohydrate donors.Angew Chem Int Ed Engl. 2013 Jun 3;52(23):6068-71. doi: 10.1002/anie.201301264. Epub 2013 Apr 22. Angew Chem Int Ed Engl. 2013. PMID: 23609997 Free PMC article.
-
Neoglycosylation and neoglycorandomization: Enabling tools for the discovery of novel glycosylated bioactive probes and early stage leads.Medchemcomm. 2014 Aug 1;5(8):1036-1047. doi: 10.1039/C4MD00117F. Medchemcomm. 2014. PMID: 25071927 Free PMC article.
References
-
- Lasseur R, Grandemange A, Longin-Sauvageon C, Berny P, Benoit E. Pestic. Biochem. Physiol. 2007;88:203–208.
- Oldenburg J, Watzka M, Rost S, Müller CR. J. Thromb. Haemost. 2007;5:1–6. - PubMed
-
- Bobek V, Kovarík J. Biomed. Pharmacother. 2004;58:213–219. - PubMed
-
- Weitz IC, Liebman HA. Semin. Thromb. Hemost. 2007;33:695–698. - PubMed
-
- Jung J-C, Park O-S. Molecules. 2009;14:4790–4803.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

