Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions
- PMID: 21714101
- PMCID: PMC3192254
- DOI: 10.1002/dneu.20931
Regulation of axonal outgrowth and pathfinding by integrin-ECM interactions
Abstract
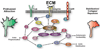
Developing neurons use a combination of guidance cues to assemble a functional neural network. A variety of proteins immobilized within the extracellular matrix (ECM) provide specific binding sites for integrin receptors on neurons. Integrin receptors on growth cones associate with a number of cytosolic adaptor and signaling proteins that regulate cytoskeletal dynamics and cell adhesion. Recent evidence suggests that soluble growth factors and classic axon guidance cues may direct axon pathfinding by controlling integrin-based adhesion. Moreover, because classic axon guidance cues themselves are immobilized within the ECM and integrins modulate cellular responses to many axon guidance cues, interactions between activated receptors modulate cell signals and adhesion. Ultimately, growth cones control axon outgrowth and pathfinding behaviors by integrating distinct biochemical signals to promote the proper assembly of the nervous system. In this review, we discuss our current understanding how ECM proteins and their associated integrin receptors control neural network formation.
Copyright © 2011 Wiley Periodicals, Inc.
Figures


References
-
- Adams DN, Kao EY, Hypolite CL, Distefano MD, Hu WS, Letourneau PC. Growth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptide. J Neurobiol. 2005;62:134–147. - PubMed
-
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

