Bayesian enrichment strategies for randomized discontinuation trials
- PMID: 21714780
- PMCID: PMC3667626
- DOI: 10.1111/j.1541-0420.2011.01623.x
Bayesian enrichment strategies for randomized discontinuation trials
Abstract
We propose optimal choice of the design parameters for random discontinuation designs (RDD) using a Bayesian decision-theoretic approach. We consider applications of RDDs to oncology phase II studies evaluating activity of cytostatic agents. The design consists of two stages. The preliminary open-label stage treats all patients with the new agent and identifies a possibly sensitive subpopulation. The subsequent second stage randomizes, treats, follows, and compares outcomes among patients in the identified subgroup, with randomization to either the new or a control treatment. Several tuning parameters characterize the design: the number of patients in the trial, the duration of the preliminary stage, and the duration of follow-up after randomization. We define a probability model for tumor growth, specify a suitable utility function, and develop a computational procedure for selecting the optimal tuning parameters.
© 2011, The International Biometric Society.
Figures

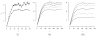
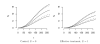
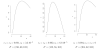
Comment in
-
Discussion of the article by Trippa, Rosner, and Müller on Bayesian enrichment strategies for randomized discontinuation trials.Biometrics. 2012 Mar;68(1):212-4; discussion 224-5. doi: 10.1111/j.1541-0420.2011.01624.x. Epub 2011 Jun 29. Biometrics. 2012. PMID: 21714781
-
Optimal Bayesian enrichment: a view from the closet.Biometrics. 2012 Mar;68(1):215-6; discussion 224-5. doi: 10.1111/j.1541-0420.2011.01625.x. Epub 2011 Jun 29. Biometrics. 2012. PMID: 21714782 No abstract available.
-
Discussion: “Bayesian enrichment strategies for randomized discontinuation trials”.Biometrics. 2012 Mar;68(1):217-8; discussion 224-5. doi: 10.1111/j.1541-0420.2011.01626.x. Epub 2011 Jun 29. Biometrics. 2012. PMID: 21714783 No abstract available.
-
Discussion of Trippa, Rosner, and Müller--Bayesian enrichment strategies for randomized discontinuation trials.Biometrics. 2012 Mar;68(1):219-23; discussion 224-5. doi: 10.1111/j.1541-0420.2011.01627.x. Epub 2011 Jun 29. Biometrics. 2012. PMID: 21714784 No abstract available.
References
-
- Albano G, Giorno V. A stochastic model in tumor growth. Journal of Theoretical Biology. 2006;241:329–336. - PubMed
-
- Albano G, Giorno V. On the first exit time problem for a Gompertz-type tumor growth. Lecture Notes in Computer Science. 2009;5717:329–336.
-
- Bursham KP, Anderson DR. Model Selection and Inference. Springer-Verlag; New York: 1998.
-
- Capra W. Comparing the power of the discontinuation design to that of the classic randomized design on time-to-event endpoints. Controlled Clinical Trials. 2004;25:168–177. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

