Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans
- PMID: 21714827
- PMCID: PMC3979351
- DOI: 10.1111/j.1750-3639.2011.00513.x
Widespread τ and amyloid-β pathology many years after a single traumatic brain injury in humans
Abstract
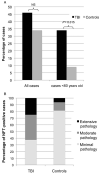
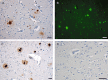
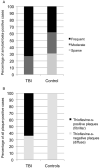
While a history of a single traumatic brain injury (TBI) is associated with the later development of syndromes of cognitive impairment such as Alzheimer's disease, the long-term pathology evolving after single TBI is poorly understood. However, a progressive tauopathy, chronic traumatic encephalopathy, is described in selected cohorts with a history of repetitive concussive/mild head injury. Here, post-mortem brains from long-term survivors of just a single TBI (1-47 years survival; n=39) vs. uninjured, age-matched controls (n=47) were examined for neurofibrillary tangles (NFTs) and amyloid-β (Aβ) plaques using immunohistochemistry and thioflavine-S staining. Detailed maps of findings permitted classification of pathology using semiquantitative scoring systems. NFTs were exceptionally rare in young, uninjured controls, yet were abundant and widely distributed in approximately one-third of TBI cases. In addition, Aβ-plaques were found in a greater density following TBI vs. controls. Moreover, thioflavine-S staining revealed that while all plaque-positive control cases displayed predominantly diffuse plaques, 64% of plaque-positive TBI cases displayed predominantly thioflavine-S-positive plaques or a mixed thioflavine-S-positive/diffuse pattern. These data demonstrate that widespread NFT and Aβ plaque pathologies are present in up to a third of patients following survival of a year or more from a single TBI. This suggests that a single TBI induces long-term neuropathological changes akin to those found in neurodegenerative disease.
© 2011 The Authors. Brain Pathology © 2011 International Society of Neuropathology.
Figures

References
-
- Braak H, Braak E (1990) Neurofibrillary changes confined to the entorhinal region and an abundance of cortical amyloid in cases of presenile and senile dementia. Acta Neuropathol 80:479–486. - PubMed
-
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82:239–259. - PubMed
-
- Braak H, Braak E (1997) Frequency of stages of Alzheimer‐related lesions in different age categories. Neurobiol Aging 18:351–357. - PubMed
-
- Buee L, Hof PR, Bouras C, Delacourte A, Perl DP, Morrison JH, Fillit HM (1994) Pathological alterations of the cerebral microvasculature in Alzheimer's disease and related dementing disorders. Acta Neuropathol 87:469–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

