Shortening of treatment duration in patients with chronic hepatitis C genotype 2 and 3 - impact of ribavirin dose - a randomized multicentre trial
- PMID: 21714878
- PMCID: PMC3141520
- DOI: 10.1186/1756-0500-4-220
Shortening of treatment duration in patients with chronic hepatitis C genotype 2 and 3 - impact of ribavirin dose - a randomized multicentre trial
Abstract
Background: Chronic hepatitis C (CHC) Patients, infected with genotype (GT) 2 or 3 are treated with Peg-IFN and ribavirin (RBV) (800 mg/day) for 24 weeks. Treatment duration can be shortened to 12-16 weeks if a higher dose of RBV (1.000/1.200 mg/day) was used without considerable loss of responsiveness or increased risk of relapse. Previously we have shown that in patients with CHC, GT 2/3 RBV can be reduced to 400 mg/day if administered for 24 weeks without an increase in relapse rates. Therefore we investigated the efficacy of a reduced RBV dosage of 400 mg/day with shorter treatment duration (16 weeks).
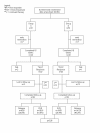
Methods: Treatment naïve patients with CHC, GT 2/3 were randomized to receive 180 μg peginterferonα2a/week in combination with either 800 (group C) or 400 mg/d (group D) for 16 weeks. The primary endpoint was SVR.
Results: 12 months after the first patient was randomized a inferior outcome of group D as compared to group C was noted, therefore the study was terminated. At study termination 89 patients were enrolled (group C: 31, D: 51). The SVR rate was statistically different in the two study groups with 51.6% in group C and 28.4% in group D (p = 0.038). Patients with low viral load had higher SVR rates (C: 67%, D: 33%) than those with high viral load (C: 33%, D: 21%).
Conclusion: Both treatment duration and the dose of RBV play a major role to optimize outcome of patients with GT3. If one intends to shorten the treatment weight based RBV dose should be used, if lower RBV doses are used patients should be treated for at least 24 weeks as. A treatment regimen with a reduced RBV dosage and shortened treatment duration is associated with low SVR rates due to high relapse rates.
Trial registration: NCT01258101.
Figures
Similar articles
-
Weight affect relapse rates in latinos with genotype 2/3 chronic hepatitis C (CHC) treated with peg IFN alfa-2a (Pegasys) 180 mcg/week and 800 mg daily of ribavirin for 24 weeks.J Med Virol. 2008 Sep;80(9):1576-80. doi: 10.1002/jmv.21253. J Med Virol. 2008. PMID: 18649339
-
Phase III results of Boceprevir in treatment naïve patients with chronic hepatitis C genotype 1.Liver Int. 2012 Feb;32 Suppl 1:27-31. doi: 10.1111/j.1478-3231.2011.02725.x. Liver Int. 2012. PMID: 22212568 Review.
-
Pharmacokinetics-Based Adjusted Versus Standard Dose of Ribavirin Does Not Improve Virologic Response Rates in Chronic Hepatitis C Genotype 4 Patients: A Randomized Controlled Trial.J Interferon Cytokine Res. 2017 Nov;37(11):488-493. doi: 10.1089/jir.2017.0054. J Interferon Cytokine Res. 2017. PMID: 29135370 Clinical Trial.
-
[Association between ribavirin plasma concentration and sustained virologic response in treatment of patients with genotype 1b chronic hepatitis C with pegylated interferon-α-2b and ribavirin].Zhonghua Gan Zang Bing Za Zhi. 2016 Mar 20;24(3):175-80. doi: 10.3760/cma.j.issn.1007-3418.2016.03.004. Zhonghua Gan Zang Bing Za Zhi. 2016. PMID: 27095759 Chinese.
-
Hematological Adverse events and Sustained Viral Response in Children Undergoing Therapy for Chronic Hepatitis C Infection.Hepat Mon. 2011 Dec;11(12):968-74. doi: 10.5812/kowsar.1735143X.789. Epub 2011 Dec 20. Hepat Mon. 2011. PMID: 22368680 Free PMC article.
References
-
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. - DOI - PubMed
-
- Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M, Zeuzem S, Pockros PJ, Lin A, Ackrill AM. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–355. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/S0140-6736(01)06102-5. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous