How Can the Evidence from Global Large-scale Clinical Trials for Cardiovascular Diseases be Improved?
- PMID: 21714928
- PMCID: PMC3224457
- DOI: 10.1186/1756-0500-4-222
How Can the Evidence from Global Large-scale Clinical Trials for Cardiovascular Diseases be Improved?
Abstract
Background: Clinical investigations are important for obtaining evidence to improve medical treatment. Large-scale clinical trials with thousands of participants are particularly important for this purpose in cardiovascular diseases. Conducting large-scale clinical trials entails high research costs. This study sought to investigate global trends in large-scale clinical trials in cardiovascular diseases.
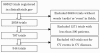
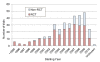
Findings: We searched for trials using clinicaltrials.gov (URL: http://www.clinicaltrials.gov/) using the key words 'cardio' and 'event' in all fields on 10 April, 2010. We then selected trials with 300 or more participants examining cardiovascular diseases. The search revealed 344 trials that met our criteria. Of 344 trials, 71% were randomized controlled trials, 15% involved more than 10,000 participants, and 59% were funded by industry. In RCTs whose results were disclosed, 55% of industry-funded trials and 25% of non-industry funded trials reported statistically significant superiority over control (p = 0.012, 2-sided Fisher's exact test).
Conclusions: Our findings highlighted concerns regarding potential bias related to funding sources, and that researchers should be aware of the importance of trial information disclosures and conflicts of interest. We should keep considering management and training regarding information disclosures and conflicts of interest for researchers. This could lead to better clinical evidence and further improvements in the development of medical treatment worldwide.
Figures
References
-
- Anon. Evaluation of drug treatment in mild hypertension: VA-NHLBI feasibility trial. Plan and preliminary results of a two-year feasibility trial for a multicenter intervention study to evaluate the benefits versus the disadvantages of treating mild hypertension. Prepared for the Veterans Administration-National Heart, Lung, and Blood Institute Study Group for Evaluating Treatment in Mild Hypertension. Ann N Y Acad Sci. 1978;304:267–292. doi: 10.1111/j.1749-6632.1978.tb25604.x. - DOI - PubMed
-
- Sawata H, Tsutani K. How do we Interpret the Evidences from Large-scale Clinical Trials of Cardiovascular Diseases for the Development of Clinical Practice Guidelines? The 75th Annual Scientific Meeting of the Japanese Circulation Society. Circ J. 2008;72(Suppl 1):63.
LinkOut - more resources
Full Text Sources
Miscellaneous