Evaluating the value of number of cycles of docetaxel and prednisone in men with metastatic castration-resistant prostate cancer
- PMID: 21715086
- PMCID: PMC3483076
- DOI: 10.1016/j.eururo.2011.06.034
Evaluating the value of number of cycles of docetaxel and prednisone in men with metastatic castration-resistant prostate cancer
Abstract
Background: The optimal number of 3-wk docetaxel plus prednisone (DP) cycles for metastatic castration-resistant prostate cancer (mCRPC) is unclear.
Objective: A retrospective analysis of two clinical trials was performed to evaluate the association of the number of cycles with overall survival (OS).
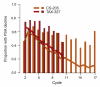
Design, setting, and participants: An exploratory analysis compared outcomes of 332 men who received DP in the TAX-327 trial, which stipulated up to 10 cycles, and 220 men who received DP in CS-205, a randomized phase 2 trial comparing DP plus AT-101 (bcl-2 inhibitor) versus DP plus placebo, which allowed up to 17 cycles.
Measurements: Patients who completed 10 cycles of DP without progression in both trials were included. Men in both arms of CS-205 were combined for analysis, as no significant differences in outcomes were observed. OS was estimated from the date of cycle 10 docetaxel infusion.
Results and limitations: The number of men receiving 10 cycles was similar (p=0.26) in the two trials (166 [50.0%] in TAX-327 vs 99 [45.0%] in CS-205; the latter group received a median of five additional cycles). Six- and 12-mo estimated survival after cycle 10 was 92.2% (95% confidence interval [CI], 86.9-95.4%) and 74.6% (CI, 67.2-80.5%) in TAX-327, compared with 92.8% (CI, 85.5-96.5) and 63.4% (CI, 51.8-72.9%) in CS-205. Subanalyses suggested that <10 cycles may have a negative impact and prostate-specific antigen (PSA) declines at cycle 10 may carry a favorable impact. The significance of continued PSA declines up to 17 cycles is unclear. Limitations of a retrospective analysis apply.
Conclusions: A survival benefit was not detected with >10 cycles of DP in men with mCRPC in this retrospective hypothesis-generating analysis.
Copyright © 2011 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Figures

Comment in
-
Re: Gregory R. Pond, Andrew J. Armstrong, Brian A. Wood, et al. Evaluating the value of number of cycles of docetaxel and prednisone in men with metastatic castration-resistant prostate cancer. Eur Urol 2012;61:363-9.Eur Urol. 2012 Feb;61(2):e3; author reply e4-5. doi: 10.1016/j.eururo.2011.07.056. Epub 2011 Aug 3. Eur Urol. 2012. PMID: 21824719 No abstract available.
References
-
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. - PubMed
-
- Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. - PubMed
-
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. - PubMed
-
- Sonpavde G, Matveev V, Burke J, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. J Clin Oncol. 2011;29(Suppl 7) abstract 125. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

