AF1q/MLLT11 regulates the emergence of human prothymocytes through cooperative interaction with the Notch signaling pathway
- PMID: 21715312
- PMCID: PMC3158713
- DOI: 10.1182/blood-2011-01-333179
AF1q/MLLT11 regulates the emergence of human prothymocytes through cooperative interaction with the Notch signaling pathway
Abstract
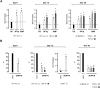
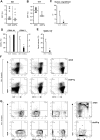
The mechanisms regulating the emergence of BM prothymocytes remain poorly characterized. Genome-wide transcriptome analyses looking for genes expressed in human prothymocytes led to the identification of AF1q/MLLT11 as a candidate gene conceivably involved in this process. Analysis of AF1q protein subcellular localization and intracellular trafficking showed that despite pronounced karyophily, it was subjected to constitutive nuclear export followed by ubiquitin-mediated degradation in the centrosomal area. Using in vitro assays based on either forced expression or shRNA-mediated silencing of AF1q, we provide evidence that the protein promotes T- over B-cell differentiation in multipotent hematopoietic progenitors. At the molecular level, AF1q confers to multipotent progenitors an increased susceptibility to Delta-like/Notch-mediated signaling. Consistent with these findings, enforced AF1q expression in humanized mice fosters the emergence of BM CD34(+)CD7(+) prothymocytes, enhances subsequent thymus colonization, and accelerates intrathymic T-cell development. In contrast, AF1q silencing provokes a global shift of BM lymphopoiesis toward the B-cell lineage, hinders prothymocyte development, inhibits thymus colonization, and leads to intrathymic accumulation of B cells. Our results indicate that AF1q cooperates with the Notch signaling pathway to foster the emergence of BM prothymocytes and drive subsequent intrathymic specification toward the T-cell lineage.
Figures





Similar articles
-
Dynamics of human prothymocytes and xenogeneic thymopoiesis in hematopoietic stem cell-engrafted nonobese diabetic-SCID/IL-2rγnull mice.J Immunol. 2012 Aug 15;189(4):1648-60. doi: 10.4049/jimmunol.1201251. Epub 2012 Jul 13. J Immunol. 2012. PMID: 22798679
-
Molecular dissection of prethymic progenitor entry into the T lymphocyte developmental pathway.J Immunol. 2007 Jul 1;179(1):421-38. doi: 10.4049/jimmunol.179.1.421. J Immunol. 2007. PMID: 17579063
-
Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes.J Immunol. 2010 Jul 15;185(2):867-76. doi: 10.4049/jimmunol.1000782. Epub 2010 Jun 14. J Immunol. 2010. PMID: 20548034
-
Regulation of intrathymic T-cell development by Lunatic Fringe- Notch1 interactions.Immunol Rev. 2006 Feb;209:76-94. doi: 10.1111/j.0105-2896.2006.00360.x. Immunol Rev. 2006. PMID: 16448535 Review.
-
Pathways from hematopoietic stem cells to thymocytes.Curr Opin Immunol. 1995 Apr;7(2):176-87. doi: 10.1016/0952-7915(95)80002-6. Curr Opin Immunol. 1995. PMID: 7546377 Review.
Cited by
-
AF1q: a novel mediator of basal and 4-HPR-induced apoptosis in ovarian cancer cells.PLoS One. 2012;7(6):e39968. doi: 10.1371/journal.pone.0039968. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22761939 Free PMC article.
-
RE1 silencing transcription factor (REST) negatively regulates ALL1-fused from chromosome 1q (AF1q) gene transcription.BMC Mol Biol. 2015 Sep 5;16:15. doi: 10.1186/s12867-015-0043-7. BMC Mol Biol. 2015. PMID: 26341630 Free PMC article.
-
AF1q is a novel TCF7 co-factor which activates CD44 and promotes breast cancer metastasis.Oncotarget. 2015 Aug 21;6(24):20697-710. doi: 10.18632/oncotarget.4136. Oncotarget. 2015. PMID: 26079538 Free PMC article.
-
MLLT11 Regulates Endometrial Stroma Cell Adhesion, Proliferation and Survival in Ectopic Lesions of Women with Advanced Endometriosis.Int J Mol Sci. 2023 Dec 28;25(1):439. doi: 10.3390/ijms25010439. Int J Mol Sci. 2023. PMID: 38203610 Free PMC article.
-
Identification of the novel prognostic biomarker, MLLT11, reveals its relationship with immune checkpoint markers in glioma.Front Oncol. 2022 Aug 12;12:889351. doi: 10.3389/fonc.2022.889351. eCollection 2022. Front Oncol. 2022. PMID: 36033495 Free PMC article.
References
-
- Awong G, Herer E, Surh CD, Dick JE, La Motte-Mohs RN, Zuniga-Pflucker JC. Characterization in vitro and engraftment potential in vivo of human progenitor T cells generated from hematopoietic stem cells. Blood. 2009;114(5):972–982. - PubMed
-
- Haddad R, Guimiot F, Six E, et al. Dynamics of thymus-colonizing cells during human development. Immunity. 2006;24(2):217–230. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

