Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing
- PMID: 21715336
- PMCID: PMC3191003
- DOI: 10.1074/jbc.M111.236802
Partial requirement of endothelin receptor B in spiral ganglion neurons for postnatal development of hearing
Abstract
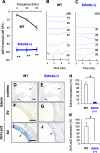
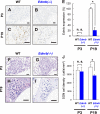
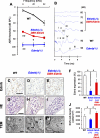
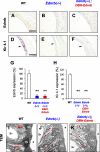
Impairments of endothelin receptor B (Ednrb/EDNRB) cause the development of Waardenburg-Shah syndrome with congenital hearing loss, hypopigmentation, and megacolon disease in mice and humans. Hearing loss in Waardenburg-Shah syndrome has been thought to be caused by an Ednrb-mediated congenital defect of melanocytes in the stria vascularis (SV) of inner ears. Here we show that Ednrb expressed in spiral ganglion neurons (SGNs) in inner ears is required for postnatal development of hearing in mice. Ednrb protein was expressed in SGNs from WT mice on postnatal day 19 (P19), whereas it was undetectable in SGNs from WT mice on P3. Correspondingly, Ednrb homozygously deleted mice (Ednrb(-/-) mice) with congenital hearing loss showed degeneration of SGNs on P19 but not on P3. The congenital hearing loss involving neurodegeneration of SGNs as well as megacolon disease in Ednrb(-/-) mice were markedly improved by introducing an Ednrb transgene under control of the dopamine β-hydroxylase promoter (Ednrb(-/-);DBH-Ednrb mice) on P19. Neither defects of melanocytes nor hypopigmentation in the SV and skin in Ednrb(-/-) mice was rescued in the Ednrb(-/-);DBH-Ednrb mice. Thus, the results of this study indicate a novel role of Ednrb expressed in SGNs distinct from that in melanocytes in the SV contributing partially to postnatal hearing development.
Figures

References
-
- Pardono E., van Bever Y., van den Ende J., Havrenne P. C., Iughetti P., Maestrelli S. R., Costa F. O., Richieri-Costa A., Frota-Pessoa O., Otto P. A. (2003) Am. J. Med. Genet. A 117A, 223–235 - PubMed
-
- Pingault V., Bondurand N., Kuhlbrodt K., Goerich D. E., Préhu M. O., Puliti A., Herbarth B., Hermans-Borgmeyer I., Legius E., Matthijs G., Amiel J., Lyonnet S., Ceccherini I., Romeo G., Smith J. C., Read A. P., Wegner M., Goossens M. (1998) Nat. Genet. 18, 171–173 - PubMed
-
- Edery P., Attié T., Amiel J., Pelet A., Eng C., Hofstra R. M., Martelli H., Bidaud C., Munnich A., Lyonnet S. (1996) Nat. Genet. 12, 442–444 - PubMed
-
- Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. (1994) Cell 79, 1257–1266 - PubMed
-
- Hosoda K., Hammer R. E., Richardson J. A., Baynash A. G., Cheung J. C., Giaid A., Yanagisawa M. (1994) Cell 79, 1267–1276 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

