Translation-competent 48S complex formation on HCV IRES requires the RNA-binding protein NSAP1
- PMID: 21715376
- PMCID: PMC3177222
- DOI: 10.1093/nar/gkr509
Translation-competent 48S complex formation on HCV IRES requires the RNA-binding protein NSAP1
Abstract
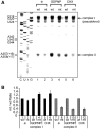
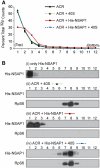
Translation of many cellular and viral mRNAs is directed by internal ribosomal entry sites (IRESs). Several proteins that enhance IRES activity through interactions with IRES elements have been discovered. However, the molecular basis for the IRES-activating function of the IRES-binding proteins remains unknown. Here, we report that NS1-associated protein 1 (NSAP1), which augments several cellular and viral IRES activities, enhances hepatitis C viral (HCV) IRES function by facilitating the formation of translation-competent 48S ribosome-mRNA complex. NSAP1, which is associated with the solvent side of the 40S ribosomal subunit, enhances 80S complex formation through correct positioning of HCV mRNA on the 40S ribosomal subunit. NSAP1 seems to accomplish this positioning function by directly binding to both a specific site in the mRNA downstream of the initiation codon and a 40S ribosomal protein (or proteins).
Figures




References
-
- Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

