Flexible tethering of primase and DNA Pol α in the eukaryotic primosome
- PMID: 21715379
- PMCID: PMC3185431
- DOI: 10.1093/nar/gkr534
Flexible tethering of primase and DNA Pol α in the eukaryotic primosome
Erratum in
- Nucleic Acids Res. 2012 May;40(10):4726
Abstract
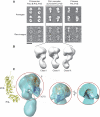
The Pol α/primase complex or primosome is the primase/polymerase complex that initiates nucleic acid synthesis during eukaryotic replication. Within the primosome, the primase synthesizes short RNA primers that undergo limited extension by Pol α. The resulting RNA-DNA primers are utilized by Pol δ and Pol ε for processive elongation on the lagging and leading strands, respectively. Despite its importance, the mechanism of RNA-DNA primer synthesis remains poorly understood. Here, we describe a structural model of the yeast primosome based on electron microscopy and functional studies. The 3D architecture of the primosome reveals an asymmetric, dumbbell-shaped particle. The catalytic centers of primase and Pol α reside in separate lobes of high relative mobility. The flexible tethering of the primosome lobes increases the efficiency of primer transfer between primase and Pol α. The physical organization of the primosome suggests that a concerted mechanism of primer hand-off between primase and Pol α would involve coordinated movements of the primosome lobes. The first three-dimensional map of the eukaryotic primosome at 25 Å resolution provides an essential structural template for understanding initiation of eukaryotic replication.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

