The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism
- PMID: 21715478
- PMCID: PMC3165846
- DOI: 10.1128/JVI.00587-11
The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism
Abstract
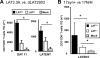
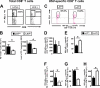
Following ocular herpes simplex virus 1 (HSV-1) infection of C57BL/6 mice, HSV-specific (HSV-gB(498-505) tetramer(+)) CD8(+) T cells are induced, selectively retained in latently infected trigeminal ganglia (TG), and appear to decrease HSV-1 reactivation. The HSV-1 latency-associated transcript (LAT) gene, the only viral gene that is abundantly transcribed during latency, increases reactivation. Previously we found that during latency with HSV-1 strain McKrae-derived viruses, more of the total TG resident CD8 T cells expressed markers of exhaustion with LAT(+) virus compared to LAT(-) virus. Here we extend these findings to HSV-1 strain 17syn+-derived LAT(+) and LAT(-) viruses and to a virus expressing just the first 20% of LAT. Thus, the previous findings were not an artifact of HSV-1 strain McKrae, and the LAT function involved mapped to the first 1.5 kb of LAT. Importantly, to our knowledge, we show here for the first time that during LAT(+) virus latency, most of the HSV-1-specific TG resident CD8 T cells were functionally exhausted, as judged by low cytotoxic function and decreased gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) production. This resulted in LAT(-) TG having more functional HSV-gB(498-505) tetramer(+) CD8(+) T cells compared to LAT(+) TG. In addition, LAT expression, in the absence of other HSV-1 gene products, appeared to be able to directly or indirectly upregulate both PD-L1 and major histocompatibility complex class I (MHC-I) on mouse neuroblastoma cells (Neuro2A). These findings may constitute a novel immune evasion mechanism whereby the HSV-1 LAT directly or indirectly promotes functional exhaustion (i.e., dysfunction) of HSV-specific CD8(+) T cells in latently infected TG, resulting in increased virus reactivation.
Figures




Similar articles
-
The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells.Viral Immunol. 2012 Jun;25(3):204-15. doi: 10.1089/vim.2011.0091. Epub 2012 Apr 18. Viral Immunol. 2012. PMID: 22512280 Free PMC article.
-
The Herpes Simplex Virus Latency-Associated Transcript Gene Is Associated with a Broader Repertoire of Virus-Specific Exhausted CD8+ T Cells Retained within the Trigeminal Ganglia of Latently Infected HLA Transgenic Rabbits.J Virol. 2016 Mar 28;90(8):3913-3928. doi: 10.1128/JVI.02450-15. Print 2016 Apr. J Virol. 2016. PMID: 26842468 Free PMC article.
-
The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus 1.J Virol. 2011 May;85(9):4184-97. doi: 10.1128/JVI.02290-10. Epub 2011 Feb 9. J Virol. 2011. PMID: 21307196 Free PMC article.
-
CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation.J Neurovirol. 2011 Dec;17(6):528-34. doi: 10.1007/s13365-011-0062-1. Epub 2011 Dec 8. J Neurovirol. 2011. PMID: 22161682 Review.
-
Control of HSV-1 latency in human trigeminal ganglia--current overview.J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review.
Cited by
-
The herpes simplex virus type 1 latency-associated transcript inhibits phenotypic and functional maturation of dendritic cells.Viral Immunol. 2012 Jun;25(3):204-15. doi: 10.1089/vim.2011.0091. Epub 2012 Apr 18. Viral Immunol. 2012. PMID: 22512280 Free PMC article.
-
Expression of Murine CD80 by Herpes Simplex Virus 1 in Place of Latency-Associated Transcript (LAT) Can Compensate for Latency Reactivation and Anti-apoptotic Functions of LAT.J Virol. 2020 Feb 28;94(6):e01798-19. doi: 10.1128/JVI.01798-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852788 Free PMC article.
-
The Absence of Lymphotoxin-α, a Herpesvirus Entry Mediator (HVEM) Ligand, Affects Herpes Simplex Virus 1 Infection In Vivo Differently than the Absence of Other HVEM Cellular Ligands.J Virol. 2019 Jul 30;93(16):e00707-19. doi: 10.1128/JVI.00707-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31142672 Free PMC article.
-
Asymptomatic memory CD8+ T cells: from development and regulation to consideration for human vaccines and immunotherapeutics.Hum Vaccin Immunother. 2014;10(4):945-63. doi: 10.4161/hv.27762. Epub 2014 Feb 5. Hum Vaccin Immunother. 2014. PMID: 24499824 Free PMC article. Review.
-
Upregulation of Multiple CD8+ T Cell Exhaustion Pathways Is Associated with Recurrent Ocular Herpes Simplex Virus Type 1 Infection.J Immunol. 2020 Jul 15;205(2):454-468. doi: 10.4049/jimmunol.2000131. Epub 2020 Jun 15. J Immunol. 2020. PMID: 32540992 Free PMC article.
References
-
- Aravalli R. N., Hu S., Rowen T. N., Gekker G., Lokensgard J. R. 2006. Differential apoptotic signaling in primary glial cells infected with herpes simplex virus 1. J. Neurovirol. 12:501–510 - PubMed
-
- Barber D. L., et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 - PubMed
-
- Bettahi I., et al. 2007. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest. Ophthalmol. Vis. Sci. 48:4643–4653 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY019896/EY/NEI NIH HHS/United States
- R21 AI110902/AI/NIAID NIH HHS/United States
- R03 EY014017/EY/NEI NIH HHS/United States
- R01 EY014900/EY/NEI NIH HHS/United States
- R01 EY024618/EY/NEI NIH HHS/United States
- EY013191/EY/NEI NIH HHS/United States
- EY14900/EY/NEI NIH HHS/United States
- EY14017/EY/NEI NIH HHS/United States
- R01 EY026103/EY/NEI NIH HHS/United States
- R01 EY013191/EY/NEI NIH HHS/United States
- EY019896/EY/NEI NIH HHS/United States
- R01 EY018171/EY/NEI NIH HHS/United States
- EY018171/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

