Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2
- PMID: 21715480
- PMCID: PMC3165831
- DOI: 10.1128/JVI.00836-11
Epigenetic silencing of HIV-1 by the histone H3 lysine 27 methyltransferase enhancer of Zeste 2
Abstract
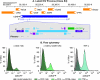
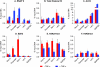
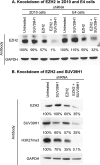
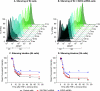
Latent HIV proviruses are silenced as the result of deacetylation and methylation of histones located at the viral long terminal repeat (LTR). Inhibition of histone deacetylases (HDACs) leads to the reemergence of HIV-1 from latency, but the contribution of histone lysine methyltransferases (HKMTs) to maintaining HIV latency remains uncertain. Chromatin immunoprecipitation experiments using latently infected Jurkat T-cell lines demonstrated that the HKMT enhancer of Zeste 2 (EZH2) was present at high levels at the LTR of silenced HIV proviruses and was rapidly displaced following proviral reactivation. Knockdown of EZH2, a key component of the Polycomb repressive complex 2 (PRC2) silencing machinery, and the enzyme which is required for trimethyl histone lysine 27 (H3K27me3) synthesis induced up to 40% of the latent HIV proviruses. In contrast, there was less than 5% induction of latent proviruses following knockdown of SUV39H1, which is required for H3K9me3 synthesis. Knockdown of EZH2 also sensitized latent proviruses to external stimuli, such as T-cell receptor stimulation, and slowed the reversion of reactivated proviruses to latency. Similarly, cell populations that responded poorly to external stimuli carried HIV proviruses that were enriched in H3K27me3 and relatively depleted in H3K9me3. Treating latently infected cells with the HKMT inhibitor 3-deazaneplanocin A, which targets EZH2, led to the reactivation of silenced proviruses, whereas chaetocin and BIX01294 showed only minimal reactivation activities. These findings suggest that PRC2-mediated silencing is an important feature of HIV latency and that inhibitors of histone methylation may play a useful role in induction strategies designed to eradicate latent HIV pools.
Figures





References
-
- Bernstein B. E., et al. 2006. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 125:315–326 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI025899/AI/NIAID NIH HHS/United States
- T32 GM008056/GM/NIGMS NIH HHS/United States
- U19 AI082608/AI/NIAID NIH HHS/United States
- DP1-DA028869/DA/NIDA NIH HHS/United States
- R01 AI067093/AI/NIAID NIH HHS/United States
- T32-GM08056/GM/NIGMS NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- DP1 DA028869/DA/NIDA NIH HHS/United States
- R01 DA030156/DA/NIDA NIH HHS/United States
- P30-AI036219/AI/NIAID NIH HHS/United States
- R01-AI025899/AI/NIAID NIH HHS/United States
- R01-AI067093/AI/NIAID NIH HHS/United States
- U19-AI082608/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

