Passive immunotherapies protect WRvFire and IHD-J-Luc vaccinia virus-infected mice from lethality by reducing viral loads in the upper respiratory tract and internal organs
- PMID: 21715493
- PMCID: PMC3165812
- DOI: 10.1128/JVI.00121-11
Passive immunotherapies protect WRvFire and IHD-J-Luc vaccinia virus-infected mice from lethality by reducing viral loads in the upper respiratory tract and internal organs
Abstract
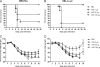
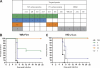
Whole-body bioimaging was employed to study the effects of passive immunotherapies on lethality and viral dissemination in BALB/c mice challenged with recombinant vaccinia viruses expressing luciferase. WRvFire and IHD-J-Luc vaccinia viruses induced lethality with similar times to death following intranasal infection, but WRvFire replicated at higher levels than IHD-J-Luc in the upper and lower respiratory tracts. Three types of therapies were tested: licensed human anti-vaccinia virus immunoglobulin intravenous (VIGIV); recombinant anti-vaccinia virus immunoglobulin (rVIG; Symphogen, Denmark), an investigational product containing a mixture of 26 human monoclonal antibodies (HuMAbs) against mature virion (MV) and enveloped virion (EV); and HuMAb compositions targeting subsets of MV or EV proteins. Bioluminescence recorded daily showed that pretreatment with VIGIV (30 mg) or with rVIG (100 μg) on day -2 protected mice from death but did not prevent viral replication at the site of inoculation and dissemination to internal organs. Compositions containing HuMAbs against MV or EV proteins were protective in both infection models at 100 μg per animal, but at 30 μg, only anti-EV antibodies conferred protection. Importantly, the t statistic of the mean total fluxes revealed that viral loads in surviving mice were significantly reduced in at least 3 sites for 3 consecutive days (days 3 to 5) postchallenge, while significant reduction for 1 or 2 days in any individual site did not confer protection. Our data suggest that reduction of viral replication at multiple sites, including respiratory tract, spleen, and liver, as monitored by whole-body bioluminescence can be used to predict the effectiveness of passive immunotherapies in mouse models.
Figures
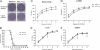

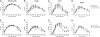



References
-
- Bell E., et al. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425–431 - PubMed
-
- Cono J., Casey C. G., Bell D. M. 2003. Smallpox vaccination and adverse reactions. Guidance for clinicians. MMWR Recomm. Rep. 52:1–28 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

