Potent and broad anti-HIV-1 activity exhibited by a glycosyl-phosphatidylinositol-anchored peptide derived from the CDR H3 of broadly neutralizing antibody PG16
- PMID: 21715497
- PMCID: PMC3165811
- DOI: 10.1128/JVI.00520-11
Potent and broad anti-HIV-1 activity exhibited by a glycosyl-phosphatidylinositol-anchored peptide derived from the CDR H3 of broadly neutralizing antibody PG16
Abstract
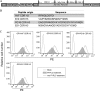
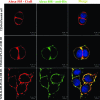
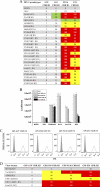
PG9 and PG16 are two recently isolated quaternary-specific human monoclonal antibodies that neutralize 70 to 80% of circulating HIV-1 isolates. The crystal structure of PG16 shows that it contains an exceptionally long CDR H3 that forms a unique stable subdomain that towers above the antibody surface to confer fine specificity. To determine whether this unique architecture of CDR H3 itself is sufficient for epitope recognition and neutralization, we cloned CDR H3 subdomains derived from human monoclonal antibodies PG16, PG9, b12, E51, and AVF and genetically linked them to a glycosyl-phosphatidylinositol (GPI) attachment signal. Each fusion gene construct is expressed and targeted to lipid rafts of plasma membranes through a GPI anchor. Moreover, GPI-CDR H3(PG16, PG9, and E51), but not GPI-CDR H3(b12 and AVF), specifically neutralized multiple clades of HIV-1 isolates with a great degree of potency when expressed on the surface of transduced TZM-bl cells. Furthermore, GPI-anchored CDR H3(PG16), but not GPI-anchored CDR H3(AVF), specifically confers resistance to HIV-1 infection when expressed on the surface of transduced human CD4(+) T cells. Finally, the CDR H3 mutations (Y100HF, D100IA, and G7) that were previously shown to compromise the neutralization activity of antibody PG16 also abolished the neutralization activity of GPI-CDR H3(PG16). Thus, we conclude that the CDR H3 subdomain of PG16 neutralizes HIV-1 when targeted to the lipid raft of the plasma membrane of HIV-1-susceptible cells and that GPI-CDR H3 can be an alternative approach for determining whether the CDR H3 of certain antibodies alone can exert epitope recognition and neutralization.
Figures

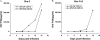
Similar articles
-
Trimeric glycosylphosphatidylinositol-anchored HCDR3 of broadly neutralizing antibody PG16 is a potent HIV-1 entry inhibitor.J Virol. 2013 Feb;87(3):1899-905. doi: 10.1128/JVI.01038-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152526 Free PMC article.
-
Crystal structure of PG16 and chimeric dissection with somatically related PG9: structure-function analysis of two quaternary-specific antibodies that effectively neutralize HIV-1.J Virol. 2010 Aug;84(16):8098-110. doi: 10.1128/JVI.00966-10. Epub 2010 Jun 10. J Virol. 2010. PMID: 20538861 Free PMC article.
-
Structure and function of broadly reactive antibody PG16 reveal an H3 subdomain that mediates potent neutralization of HIV-1.Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11483-8. doi: 10.1073/pnas.1004600107. Epub 2010 Jun 2. Proc Natl Acad Sci U S A. 2010. PMID: 20534513 Free PMC article.
-
Structural Features of Broadly Neutralizing Antibodies and Rational Design of Vaccine.Adv Exp Med Biol. 2018;1075:73-95. doi: 10.1007/978-981-13-0484-2_4. Adv Exp Med Biol. 2018. PMID: 30030790 Review.
-
Ontogeny-based immunogens for the induction of V2-directed HIV broadly neutralizing antibodies.Immunol Rev. 2017 Jan;275(1):217-229. doi: 10.1111/imr.12501. Immunol Rev. 2017. PMID: 28133797 Free PMC article. Review.
Cited by
-
Trimeric glycosylphosphatidylinositol-anchored HCDR3 of broadly neutralizing antibody PG16 is a potent HIV-1 entry inhibitor.J Virol. 2013 Feb;87(3):1899-905. doi: 10.1128/JVI.01038-12. Epub 2012 Nov 14. J Virol. 2013. PMID: 23152526 Free PMC article.
-
A Membrane-Anchored Short-Peptide Fusion Inhibitor Fully Protects Target Cells from Infections of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.J Virol. 2019 Oct 29;93(22):e01177-19. doi: 10.1128/JVI.01177-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462566 Free PMC article.
-
Engineered CD4 T cells expressing a membrane anchored viral inhibitor restrict HIV-1 through cis and trans mechanisms.Front Immunol. 2023 Sep 14;14:1167965. doi: 10.3389/fimmu.2023.1167965. eCollection 2023. Front Immunol. 2023. PMID: 37781368 Free PMC article.
-
CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection.PLoS One. 2014 Dec 26;9(12):e115987. doi: 10.1371/journal.pone.0115987. eCollection 2014. PLoS One. 2014. PMID: 25541967 Free PMC article.
-
Transmembrane domain membrane proximal external region but not surface unit-directed broadly neutralizing HIV-1 antibodies can restrict dendritic cell-mediated HIV-1 trans-infection.J Infect Dis. 2012 Apr 15;205(8):1248-57. doi: 10.1093/infdis/jis183. Epub 2012 Mar 6. J Infect Dis. 2012. PMID: 22396600 Free PMC article.
References
-
- Burton D. R., et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024–1027 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

