Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes
- PMID: 21715554
- PMCID: PMC3142064
- DOI: 10.2337/db10-1222
Superoxide production by macrophages and T cells is critical for the induction of autoreactivity and type 1 diabetes
Abstract
Objective: The role of reactive oxygen species (ROS) and their dissipation in type 1 diabetes pathogenesis have garnered considerable controversy. Our recent work has demonstrated the importance of NADPH oxidase (NOX) activity for type 1 diabetes development and modulating T-cell autoreactivity. We previously linked decreased monocyte ROS with diabetes resistance in the alloxan-resistant mouse, and NOD-Ncf1(m1J) mice with a genetic ablation of NOX activity had reduced and delayed type 1 diabetes compared with NOD mice.
Research design and methods: To determine the required cellular sources of ROS that are necessary for type 1 diabetes initiation, we used antibody depletion and adoptive transfer experiments into NOD and NOD-Scid females, respectively. After receiving treatment, female mice were monitored for hyperglycemia and overt diabetes.
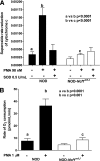
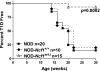
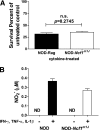
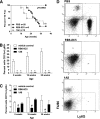
Results: Depletion of macrophages and neutrophils fully protected NOD mice from type 1 diabetes. However, elimination of neutrophils alone showed no significant reduction or delay. Type 1 diabetes induction in NOD-Scid mice by adoptive transfer with NOD-Ncf1(m1J) splenocytes was significantly delayed compared with NOD splenocytes, suggesting macrophage ROS and modulation of effector responses are critical for diabetes. The adaptive immune response was also altered by the absence of NOX activity, as purified T cells from NOD-Ncf1(m1J) mice exhibited delayed transfer kinetics. Cotransfer experiments demonstrated the defect was intrinsic to NOX-deficient CD8(+) T cells. After stimulation, cytotoxic T cells exhibited decreased effector function in the absence of superoxide production.
Conclusions: These data demonstrate that the impaired autoreactive response of NOX-deficient NOD-Ncf1(m1J) immune system results from an alteration in the antigen-presenting cell-T-cell axis rather than failure of neutrophils to act as effector cells and that ROS signaling is important for the initiation of β-cell-directed autoimmunity by T cells.
Figures


References
-
- Bouma G, Coppens JM, Mourits S, et al. Evidence for an enhanced adhesion of DC to fibronectin and a role of CCL19 and CCL21 in the accumulation of DC around the pre-diabetic islets in NOD mice. Eur J Immunol 2005;35:2386–2396 - PubMed
-
- Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity 1997;7:727–738 - PubMed
-
- Griffiths HR. ROS as signalling molecules in T cells—evidence for abnormal redox signalling in the autoimmune disease, rheumatoid arthritis. Redox Rep 2005;10:273–280 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM040586/GM/NIGMS NIH HHS/United States
- R56 DK074656/DK/NIDDK NIH HHS/United States
- GM-40586-22/GM/NIGMS NIH HHS/United States
- T32 GM008721/GM/NIGMS NIH HHS/United States
- T32 AI-007051/AI/NIAID NIH HHS/United States
- AI-056374/AI/NIAID NIH HHS/United States
- R01 GM081923/GM/NIGMS NIH HHS/United States
- DK-074656/DK/NIDDK NIH HHS/United States
- R01 DK074656/DK/NIDDK NIH HHS/United States
- T32 AI007051/AI/NIAID NIH HHS/United States
- GM-81923-03/GM/NIGMS NIH HHS/United States
- U19 AI056374/AI/NIAID NIH HHS/United States
- R01 GM040586/GM/NIGMS NIH HHS/United States
- T32 GM-008721-13/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

