Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism
- PMID: 21715610
- PMCID: PMC3132881
- DOI: 10.1523/JNEUROSCI.1460-11.2011
Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism
Abstract
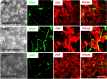
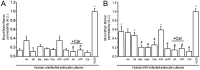
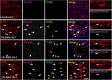
HIV infection of the CNS is an early event after primary infection, resulting in neurological complications in a significant number of individuals despite antiretroviral therapy (ART). The main cells infected with HIV within the CNS are macrophages/microglia and a small fraction of astrocytes. The role of these few infected astrocytes in the pathogenesis of neuroAIDS has not been examined extensively. Here, we demonstrate that few HIV-infected astrocytes (4.7 ± 2.8% in vitro and 8.2 ± 3.9% in vivo) compromise blood-brain barrier (BBB) integrity. This BBB disruption is due to endothelial apoptosis, misguided astrocyte end feet, and dysregulation of lipoxygenase/cyclooxygenase, BK(Ca) channels, and ATP receptor activation within astrocytes. All of these alterations in BBB integrity induced by a few HIV-infected astrocytes were gap junction dependent, as blocking these channels protected the BBB from HIV-infected astrocyte-mediated compromise. We also demonstrated apoptosis in vivo of BBB cells in contact with infected astrocytes using brain tissue sections from simian immunodeficiency virus-infected macaques as a model of neuroAIDS, suggesting an important role for these few infected astrocytes in the CNS damage seen with HIV infection. Our findings describe a novel mechanism of bystander BBB toxicity mediated by low numbers of HIV-infected astrocytes and amplified by gap junctions. This mechanism of toxicity contributes to understanding how CNS damage is spread even in the current ART era and how minimal or controlled HIV infection still results in cognitive impairment in a large population of infected individuals.
Figures


References
-
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–536. - PubMed
-
- Babas T, Vieler E, Hauer DA, Adams RJ, Tarwater PM, Fox K, Clements JE, Zink MC. Pathogenesis of SIV pneumonia: selective replication of viral genotypes in the lung. Virology. 2001;287:371–381. - PubMed
-
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomerantz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10:573–585. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
