Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system
- PMID: 21715636
- PMCID: PMC6623171
- DOI: 10.1523/JNEUROSCI.5814-10.2011
Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system
Abstract
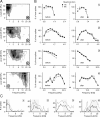
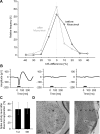
The detection of novel and therefore potentially behavioral relevant stimuli is of fundamental importance for animals. In the auditory system, stimulus-specific adaptation (SSA) resulting in stronger responses to rare compared with frequent stimuli was proposed as such a novelty detection mechanism. SSA is a now well established phenomenon found at different levels along the mammalian auditory pathway. It depends on various stimulus features, such as deviant probability, and may be an essential mechanism underlying perception of changes in sound statistics. We recorded neuronal responses from the ventral part of the medial geniculate body (vMGB) in Mongolian gerbils to determine details of the adaptation process that might indicate underlying neuronal mechanisms. Neurons in the vMGB exhibited a median spike rate change of 15.4% attributable to a fast habituation to the frequently presented standard stimulus. Accordingly, the main habituation effect could also be induced by the repetition of a few uniform tonal stimuli. The degree of habituation was frequency-specific, and comparison across simultaneously recorded units indicated that adaptation effects were apparently topographically organized. At the population level, stronger habituation effects were on average associated with the border regions of the frequency response areas. Finally, the pharmacological inactivation of the auditory cortex demonstrated that SSA in the vMGB is mainly regulated by the corticofugal system. Hence, these results indicate a more general function of SSA in the processing and analysis of auditory information than the term novelty detection suggests.
Figures






References
-
- Abel C, Kössl M. Sensitive response to low-frequency cochlear distortion products in the auditory midbrain. J Neurophysiol. 2009;101:1560–1574. - PubMed
-
- Antunes FM, Covey E, Malmierca MS. Is there stimulus-specific adaptation in the medial geniculate body of the rat? In: Lopez-Poveda EA, Palmer AR, Meddis R, editors. The neurophysiological bases of auditory perception. New York: Springer; 2010. pp. 535–544.
-
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull. 1997;42:27–37. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
