The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells
- PMID: 21715642
- PMCID: PMC3365553
- DOI: 10.1523/JNEUROSCI.1604-11.2011
The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells
Abstract
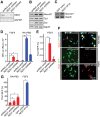
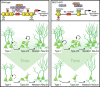
Transcriptional regulation is a critical mechanism in the birth, specification, and differentiation of granule neurons in the adult hippocampus. One of the first negative-acting transcriptional regulators implicated in vertebrate development is repressor element 1-silencing transcription/neuron-restrictive silencer factor (REST/NRSF)--thought to regulate hundreds of neuron-specific genes--yet its function in the adult brain remains elusive. Here we report that REST/NRSF is required to maintain the adult neural stem cell (NSC) pool and orchestrate stage-specific differentiation. REST/NRSF recruits CoREST and mSin3A corepressors to stem cell chromatin for the regulation of pro-neuronal target genes to prevent precocious neuronal differentiation in cultured adult NSCs. Moreover, mice lacking REST/NRSF specifically in NSCs display a transient increase in adult neurogenesis that leads to a loss in the neurogenic capacity of NSCs and eventually diminished granule neurons. Our work identifies REST/NRSF as a master negative regulator of adult NSC differentiation and offers a potential molecular target for neuroregenerative approaches.
Figures







References
-
- Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279:51163–51171. - PubMed
-
- Ballas N, Battaglioli E, Atouf F, Andres ME, Chenoweth J, Anderson ME, Burger C, Moniwa M, Davie JR, Bowers WJ, Federoff HJ, Rose DW, Rosenfeld MG, Brehm P, Mandel G. Regulation of neuronal traits by a novel transcriptional complex. Neuron. 2001;31:353–365. - PubMed
-
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
