CD8+ T cells primed in the periphery provide time-bound immune-surveillance to the central nervous system
- PMID: 21715683
- PMCID: PMC5047759
- DOI: 10.4049/jimmunol.1100695
CD8+ T cells primed in the periphery provide time-bound immune-surveillance to the central nervous system
Abstract
After vaccination, memory CD8(+) T cells migrate to different organs to mediate immune surveillance. In most nonlymphoid organs, following an infection, CD8(+) T cells differentiate to become long-lived effector-memory cells, thereby providing long-term protection against a secondary infection. In this study, we demonstrated that Ag-specific CD8(+) T cells that migrate to the mouse brain following a systemic Listeria infection do not display markers reminiscent of long-term memory cells. In contrast to spleen and other nonlymphoid organs, none of the CD8(+) T cells in the brain reverted to a memory phenotype, and all of the cells were gradually eliminated. These nonmemory phenotype CD8(+) T cells were found primarily within the choroid plexus, as well as in the cerebrospinal fluid-filled spaces. Entry of these CD8(+) T cells into the brain was governed primarily by CD49d/VCAM-1, with the majority of entry occurring in the first week postinfection. When CD8(+) T cells were injected directly into the brain parenchyma, cells that remained in the brain retained a highly activated (CD69(hi)) phenotype and were gradually lost, whereas those that migrated out to the spleen were CD69(low) and persisted long-term. These results revealed a mechanism of time-bound immune surveillance to the brain by CD8(+) T cells that do not reside in the parenchyma.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures






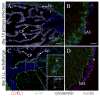

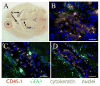
References
-
- Hayashi T, Nagai S, Fujii H, Baba Y, Ikeda E, Kawase T, Koyasu S. Critical roles of NK and CD8+ T cells in central nervous system listeriosis. J Immunol. 2009;182:6360–6368. - PubMed
-
- Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12:425–431. - PubMed
-
- Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997;9:35–43. - PubMed
-
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. - PubMed
-
- Kägi D, Ledermann B, Bürki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

