The Subthalamic Nucleus becomes a Generator of Bursts in the Dopamine-Depleted State. Its High Frequency Stimulation Dramatically Weakens Transmission to the Globus Pallidus
- PMID: 21716635
- PMCID: PMC3115486
- DOI: 10.3389/fnsys.2011.00043
The Subthalamic Nucleus becomes a Generator of Bursts in the Dopamine-Depleted State. Its High Frequency Stimulation Dramatically Weakens Transmission to the Globus Pallidus
Abstract
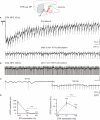
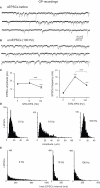
Excessive burst firing in the dopamine-depleted basal ganglia correlates with severe motor symptoms of Parkinson's disease that are attenuated by high frequency electrical stimulation of the subthalamic nucleus (STN). Here we test the hypothesis that pathological bursts in dopamine-deprived basal ganglia are generated within the STN and transmitted to globus pallidus neurons. To answer this question we recorded excitatory synaptic currents and potentials from subthalamic and pallidal neurons in the basal ganglia slice (BGS) from dopamine-depleted mice while continuously blocking GABA(A) receptors. In control mice, a single electrical stimulus delivered to the internal capsule or the rostral pole of the STN evoked a short duration, small amplitude, monosynaptic EPSC in subthalamic neurons. In contrast, in the dopamine-depleted BGS, this monosynaptic EPSC was amplified and followed by a burst of polysynaptic EPSCs that eventually reverberated three to seven times, providing a long lasting response that gave rise to bursts of EPSCs and spikes in GP neurons. Repetitive (10-120 Hz) stimulation delivered to the STN in the dopamine-depleted BGS attenuated STN-evoked bursts of EPSCs in pallidal neurons after several minutes of stimulation but only high frequency (90-120 Hz) stimulation replaced them with small amplitude EPSCs at 20 Hz. We propose that the polysynaptic pathway within the STN amplifies subthalamic responses to incoming excitation in the dopamine-depleted basal ganglia, thereby transforming the STN into a burst generator and entraining pallidal neurons in pathogenic bursting activities. High frequency stimulation of the STN prevents the transmission of this pathological activity to globus pallidus and imposes a new glutamatergic synaptic noise on pallidal neurons.
Keywords: Parkinson; basal ganglia; basal ganglia slice; burst firing; high frequency stimulation; subthalamic nucleus.
Figures


References
-
- Benazzouz A., Gao D. M., Ni Z. G., Piallat B., Bouali-Benazzouz R., Benabid A. L. (2000). Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience 99, 289–29510.1016/S0306-4522(00)00199-8 - DOI - PubMed
LinkOut - more resources
Full Text Sources

