The Stillbirth Collaborative Research Network (SCRN) placental and umbilical cord examination protocol
- PMID: 21717387
- PMCID: PMC4316371
- DOI: 10.1055/s-0031-1281509
The Stillbirth Collaborative Research Network (SCRN) placental and umbilical cord examination protocol
Abstract
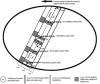
The Stillbirth Collaborative Research Network (SCRN) was organized to study the scope and causes of stillbirth (SB) in the United States. The objective of this report is to describe the approach used for the placental examination performed as part of the study. The SCRN consists of a multidisciplinary team of investigators from five clinical sites, the National Institute of Child Health and Human Development, and the Data Coordination and Analysis Center. The study is a population-based cohort and nested case-control study, with prospective enrollment of women with SB and live births (LB) at the time of delivery. Detailed and standardized postmortem examination was performed on SB and placental examination in both groups. A total of 663 women with SB and 1932 women with LB were enrolled into the case-control study. In the SB group, there were 707 fetuses. Of these cases, 654 (98.6%) had placental examination. Of these LB controls, 1804 (93.4%) had placental examination. This is the largest prospective study to include population-based SB and LB, using standardized postmortem and placental examination, medical record review, maternal interview, collection of samples, and a multidisciplinary team of investigators collaborating in the analyses. Thus it has the potential to provide high-level evidence regarding the contribution of placental abnormalities to stillbirth.
© Thieme Medical Publishers.
Figures
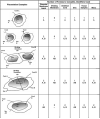

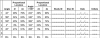
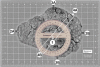
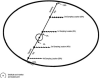

References
-
- Driscoll SG, Langston C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on Methods for Placental Examination. Arch Pathol Lab Med. 1991;115:704–708. - PubMed
-
- Kaplan C, Lowell DM, Salafia C. College of American Pathologists Conference XIX on the Examination of the Placenta: report of the Working Group on the Definition of Structural Changes Associated with Abnormal Function in the Maternal/Fetal/Placental Unit in the Second and Third Trimesters. Arch Pathol Lab Med. 1991;115:709–716. - PubMed
-
- The examination of the placenta: patient care and risk management. College of American Pathologists Conference XIX. Northfield, Illinois, September 6–7, 1990. Proceedings. Arch Pathol Lab Med. 1991;115:660–721. - PubMed
-
- Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1997;121:449–476. - PubMed
-
- Committee on Genetics ACOG Committee Opinion No. 383: Evaluation of stillbirths and neonatal deaths. Obstet Gynecol. 2007;110:963–966. - PubMed
Publication types
MeSH terms
Grants and funding
- U01-HD-45954/HD/NICHD NIH HHS/United States
- U10-HD045952/HD/NICHD NIH HHS/United States
- U10 HD045953/HD/NICHD NIH HHS/United States
- U10-HD045953/HD/NICHD NIH HHS/United States
- U10 HD045925/HD/NICHD NIH HHS/United States
- U10 HD045952/HD/NICHD NIH HHS/United States
- U10-HD045925/HD/NICHD NIH HHS/United States
- U10 HD045955/HD/NICHD NIH HHS/United States
- U10-HD045955/HD/NICHD NIH HHS/United States
- U10 HD045944/HD/NICHD NIH HHS/United States
- U01 HD045954/HD/NICHD NIH HHS/United States
- U10-HD045944/HD/NICHD NIH HHS/United States

