OX1 orexin/hypocretin receptor activation of phospholipase D
- PMID: 21718304
- PMCID: PMC3346250
- DOI: 10.1111/j.1476-5381.2011.01565.x
OX1 orexin/hypocretin receptor activation of phospholipase D
Abstract
Background and purpose: Orexin receptors potently signal to lipid messenger systems, and our previous studies have suggested that PLD would be one of these. We thus wanted to verify this by direct measurements and clarify the molecular mechanism of the coupling.
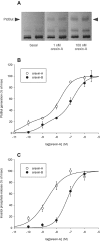
Experimental approach: Orexin receptor-mediated PLD activation was investigated in CHO cells stably expressing human OX(1) orexin receptors using [(14) C]-oleic acid-prelabelling and the transphosphatidylation assay.
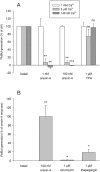
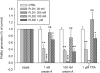
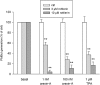
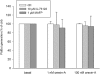
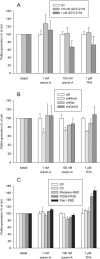
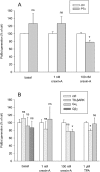
Key results: Orexin stimulation strongly increased PLD activity - even more so than the phorbol ester TPA (12-O-tetradecanoyl-phorbol-13-acetate), a highly potent activator of PLD. Both orexin and TPA responses were mediated by PLD1. Orexin-A and -B showed approximately 10-fold difference in potency, and the concentration-response curves were biphasic. Using pharmacological inhibitors and activators, both orexin and TPA were shown to signal to PLD1 via the novel PKC isoform, PKCδ. In contrast, pharmacological or molecular biological inhibitors of Rho family proteins RhoA/B/C, cdc42 and Rac did not inhibit the orexin (or the TPA) response, nor did the molecular biological inhibitors of PKD. In addition, neither cAMP elevation, Gα(i/o) nor Gβγ seemed to play an important role in the orexin response.
Conclusions and implications: Stimulation of OX(1) receptors potently activates PLD (probably PLD1) in CHO cells and this is mediated by PKCδ but not other PKC isoforms, PKDs or Rho family G-proteins. At present, the physiological significance of orexin-induced PLD activation is unknown, but this is not the first time we have identified PKCδ in orexin signalling, and thus some specific signalling cascade may exist between orexin receptors and PKCδ.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures



Similar articles
-
Arachidonic acid release mediated by OX1 orexin receptors.Br J Pharmacol. 2010 Jan;159(1):212-21. doi: 10.1111/j.1476-5381.2009.00535.x. Epub 2009 Dec 4. Br J Pharmacol. 2010. PMID: 20002100 Free PMC article.
-
Multiple phospholipase activation by OX(1) orexin/hypocretin receptors.Cell Mol Life Sci. 2008 Jun;65(12):1948-56. doi: 10.1007/s00018-008-8206-z. Cell Mol Life Sci. 2008. PMID: 18488139 Free PMC article.
-
OX1 orexin/hypocretin receptor signaling through arachidonic acid and endocannabinoid release.Mol Pharmacol. 2012 Aug;82(2):156-67. doi: 10.1124/mol.112.078063. Epub 2012 May 1. Mol Pharmacol. 2012. PMID: 22550093
-
Orexins/hypocretins: pain regulation and cellular actions.Curr Pharm Des. 2010;16(28):3089-100. doi: 10.2174/138161210793292483. Curr Pharm Des. 2010. PMID: 20687883 Review.
-
Orexins and the treatment of obesity.Eur J Pharmacol. 2002 Apr 12;440(2-3):199-212. doi: 10.1016/s0014-2999(02)01429-2. Eur J Pharmacol. 2002. PMID: 12007536 Review.
Cited by
-
Orexin receptor agonist Yan 7874 is a weak agonist of orexin/hypocretin receptors and shows orexin receptor-independent cytotoxicity.PLoS One. 2017 Jun 2;12(6):e0178526. doi: 10.1371/journal.pone.0178526. eCollection 2017. PLoS One. 2017. PMID: 28575023 Free PMC article.
-
Comparative Network Analysis of Patients with Non-Small Cell Lung Cancer and Smokers for Representing Potential Therapeutic Targets.Sci Rep. 2017 Oct 23;7(1):13812. doi: 10.1038/s41598-017-14195-1. Sci Rep. 2017. PMID: 29062084 Free PMC article.
-
Orexin Signaling: A Complex, Multifaceted Process.Front Cell Neurosci. 2022 Apr 13;16:812359. doi: 10.3389/fncel.2022.812359. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35496914 Free PMC article. Review.
-
Treatment with Gold Nanoparticles Using Cudrania tricuspidata Root Extract Induced Downregulation of MMP-2/-9 and PLD1 and Inhibited the Invasiveness of Human U87 Glioblastoma Cells.Int J Mol Sci. 2020 Feb 14;21(4):1282. doi: 10.3390/ijms21041282. Int J Mol Sci. 2020. PMID: 32074974 Free PMC article.
-
Identification and Characterization of Cannabidiol as an OX1R Antagonist by Computational and In Vitro Functional Validation.Biomolecules. 2021 Aug 1;11(8):1134. doi: 10.3390/biom11081134. Biomolecules. 2021. PMID: 34439801 Free PMC article.
References
-
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, et al. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. - PubMed
-
- Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, et al. OX1 orexin receptors activate extracellular signal-regulated kinase (ERK) in CHO cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006a;20:80–99. - PubMed
-
- Ammoun S, Lindholm D, Wootz H, Åkerman KE, Kukkonen JP. G-protein-coupled OX1 orexin/hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through p38 mitogen-/stress-activated protein kinase. J Biol Chem. 2006b;281:834–842. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

