The synthesis and evaluation of N1-(4-(2-[18F]-fluoroethyl)phenyl)-N8-hydroxyoctanediamide ([18F]-FESAHA), a PET radiotracer designed for the delineation of histone deacetylase expression in cancer
- PMID: 21718944
- PMCID: PMC3145497
- DOI: 10.1016/j.nucmedbio.2010.12.008
The synthesis and evaluation of N1-(4-(2-[18F]-fluoroethyl)phenyl)-N8-hydroxyoctanediamide ([18F]-FESAHA), a PET radiotracer designed for the delineation of histone deacetylase expression in cancer
Abstract
Introduction: Given the significant utility of suberoylanilide hydroxamic acid (SAHA) in chemotherapeutic protocols, a PET tracer that mimics the histone deacetylase (HDAC) inhibition of SAHA could be a valuable tool in the diagnosis, treatment planning and treatment monitoring of cancer. Here, we describe the synthesis, characterization and evaluation of N(1)-(4-(2-[(18)F]-fluoroethyl)phenyl)-N(8)-hydroxyoctanediamide ([(18)F]-FESAHA), a PET tracer designed for the delineation of HDAC expression in cancer.
Methods: FESAHA was synthesized and biologically characterized in vivo and in vitro. [(18)F]-FESAHA was then synthesized in high radiochemical purity, and the logP and serum stability of the radiotracer were determined. In vitro cellular uptake experiments and acute biodistribution and small-animal PET studies were performed with [(18)F]-FESAHA in mice bearing LNCaP xenografts.
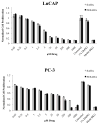
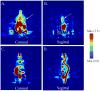
Results: [(18)F]-FESAHA was synthesized in high radiochemical purity via an innovative one-pot procedure. Enzymatic inhibition assays illustrated that FESAHA is a potent HDAC inhibitor, with IC(50) values from 3 nM to 1.7 μM against the 11 HDAC subtypes. Cell proliferation experiments revealed that the cytostatic properties of FESAHA very closely resemble those of SAHA in both LNCaP cells and PC-3 cells. Acute biodistribution and PET imaging experiments revealed tumor uptake of [(18)F]-FESAHA and substantially higher values in the small intestine, kidneys, liver and bone.
Conclusion: The significant non-tumor background uptake of [(18)F]-FESAHA presents a substantial obstacle to the use of the radiotracer as an HDAC expression imaging agent. The study at hand, however, does present a number of lessons critical to both the synthesis of hydroxamic acid containing PET radiotracers and imaging agents aimed at delineating HDAC expression.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures


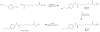
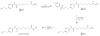

References
-
- Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. Journal of Clinical Oncology. 2005;23:3971–93. - PubMed
-
- Egger G, Liang G, Aparicio A, Jones P. Epigenetics in human diseases and prospects for epigenetic therapy. Nature. 2004;429:457–63. - PubMed
-
- Turner BM. Histone acetylation and control of gene expression. Journal of Cell Science. 1991;99:13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

