Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial
- PMID: 21719095
- PMCID: PMC3191495
- DOI: 10.1016/S0140-6736(11)60931-8
Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial
Abstract
Background: Findings of small studies have suggested that short treatments with anti-CD3 monoclonal antibodies that are mutated to reduce Fc receptor binding preserve β-cell function and decrease insulin needs in patients with recent-onset type 1 diabetes. In this phase 3 trial, we assessed the safety and efficacy of one such antibody, teplizumab.
Methods: In this 2-year trial, patients aged 8-35 years who had been diagnosed with type 1 diabetes for 12 weeks or fewer were enrolled and treated at 83 clinical centres in North America, Europe, Israel, and India. Participants were allocated (2:1:1:1 ratio) by an interactive telephone system, according to computer-generated block randomisation, to receive one of three regimens of teplizumab infusions (14-day full dose, 14-day low dose, or 6-day full dose) or placebo at baseline and at 26 weeks. The Protégé study is still underway, and patients and study staff remain masked through to study closure. The primary composite outcome was the percentage of patients with insulin use of less than 0·5 U/kg per day and glycated haemoglobin A(1c) (HbA(1C)) of less than 6·5% at 1 year. Analyses included all patients who received at least one dose of study drug. This trial is registered with ClinicalTrials.gov, number NCT00385697.
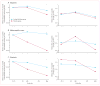
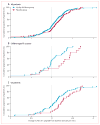
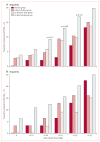
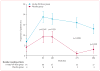
Findings: 763 patients were screened, of whom 516 were randomised to receive 14-day full-dose teplizumab (n=209), 14-day low-dose teplizumab (n=102), 6-day full-dose teplizumab (n=106), or placebo (n=99). Two patients in the 14-day full-dose group and one patient in the placebo group did not start treatment, so 513 patients were eligible for efficacy analyses. The primary outcome did not differ between groups at 1 year: 19·8% (41/207) in the 14-day full-dose group; 13·7% (14/102) in the 14-day low-dose group; 20·8% (22/106) in the 6-day full-dose group; and 20·4% (20/98) in the placebo group. 5% (19/415) of patients in the teplizumab groups were not taking insulin at 1 year, compared with no patients in the placebo group at 1 year (p=0·03). Across the four study groups, similar proportions of patients had adverse events (414/417 [99%] in the teplizumab groups vs 98/99 [99%] in the placebo group) and serious adverse events (42/417 [10%] vs 9/99 [9%]). The most common clinical adverse event in the teplizumab groups was rash (220/417 [53%] vs 20/99 [20%] in the placebo group).
Interpretation: Findings of exploratory analyses suggest that future studies of immunotherapeutic intervention with teplizumab might have increased success in prevention of a decline in β-cell function (measured by C-peptide) and provision of glycaemic control at reduced doses of insulin if they target patients early after diagnosis of diabetes and children.
Funding: MacroGenics, the Juvenile Diabetes Research Foundation, and Eli Lilly.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CH, DC, KLK, EB, SJ, KES, SK, and AGD are employees of MacroGenics and potentially own shares or options in the company. NS, JL, SA, BB, JW, and RJF received research and travel support for this study from MacroGenics. JL has received advisory board support from Johnson & Johnson. RJF has received research support within the past 3 years from Tolerx (to study otelixizumab for recent-onset type 1 diabetes); unrelated research support from the US National Institutes of Health (grants R21 HD059292 and T35 DK007405), Gabrielle’s Angel Foundation, Eli Lilly, Diamyd, Pfizer, and Novo Nordisk; and unrestricted research support from Le Bonheur Foundation (Memphis TN, USA). WH chairs the data safety and monitoring board for the BHT-3021-01 insulin plasmid trial (Bayhill Pharmaceuticals). KCH has received research support from MacroGenics, and support from the Juvenile Diabetes Foundation (grant 2008-502) for laboratory studies on patients’ samples from Protégé. RLW is a former employee of MacroGenics and holds restricted stock in the company, and is now an employee of Parexel International, the lead contract research organisation that coordinated the Protégé trial globally. SA has received speaking honoraria from Eli Lilly. SP was formerly employed by MacroGenics and received stock options while employed there. SMJ declares that he has no conflicts of interest.
Figures

Comment in
-
Anti-CD3 antibodies for type 1 diabetes: beyond expectations.Lancet. 2011 Aug 6;378(9790):459-60. doi: 10.1016/S0140-6736(11)60980-X. Epub 2011 Jun 28. Lancet. 2011. PMID: 21719098 No abstract available.
References
-
- van Belle TL, Coppieters KT, von Herrath MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev. 2011;91:79–118. - PubMed
-
- Steele C, Hagopian WA, Gitelman S, et al. Insulin secretion in type 1 diabetes. Diabetes. 2004;53:426–33. - PubMed
-
- Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC. Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes. 2006;55:3238–45. - PubMed
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. - PubMed
-
- Diabetes Prevention Trial—Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

