Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial
- PMID: 21719096
- PMCID: PMC3462593
- DOI: 10.1016/S0140-6736(11)60886-6
Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial
Abstract
Background: The immunopathogenesis of type 1 diabetes mellitus is associated with T-cell autoimmunity. To be fully active, immune T cells need a co-stimulatory signal in addition to the main antigen-driven signal. Abatacept modulates co-stimulation and prevents full T-cell activation. We evaluated the effect of abatacept in recent-onset type 1 diabetes.
Methods: In this multicentre, double-blind, randomised controlled trial, patients aged 6-45 years recently diagnosed with type 1 diabetes were randomly assigned (2:1) to receive abatacept (10 mg/kg, maximum 1000 mg per dose) or placebo infusions intravenously on days 1, 14, 28, and monthly for a total of 27 infusions over 2 years. Computer-generated permuted block randomisation was used, with a block size of 3 and stratified by participating site. Neither patients nor research personnel were aware of treatment assignments. The primary outcome was baseline-adjusted geometric mean 2-h area-under-the-curve (AUC) serum C-peptide concentration after a mixed-meal tolerance test at 2 years' follow-up. Analysis was by intention to treat for all patients for whom data were available. This trial is registered at ClinicalTrials.gov, NCT00505375.
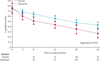
Findings: 112 patients were assigned to treatment groups (77 abatacept, 35 placebo). Adjusted C-peptide AUC was 59% (95% CI 6·1-112) higher at 2 years with abatacept (n=73, 0·378 nmol/L) than with placebo (n=30, 0·238 nmol/L; p=0·0029). The difference between groups was present throughout the trial, with an estimated 9·6 months' delay (95% CI 3·47-15·6) in C-peptide reduction with abatacept. There were few infusion-related adverse events (36 reactions occurred in 17 [22%] patients on abatacept and 11 reactions in six [17%] on placebo). There was no increase in infections (32 [42%] patients on abatacept vs 15 [43%] on placebo) or neutropenia (seven [9%] vs five [14%]).
Interpretation: Co-stimulation modulation with abatacept slowed reduction in β-cell function over 2 years. The beneficial effect suggests that T-cell activation still occurs around the time of clinical diagnosis of type 1 diabetes. Yet, despite continued administration of abatacept over 24 months, the decrease in β-cell function with abatacept was parallel to that with placebo after 6 months of treatment, causing us to speculate that T-cell activation lessens with time. Further observation will establish whether the beneficial effect continues after cessation of abatacept infusions.
Funding: US National Institutes of Health.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr. Orban reports serving on the Data Safety Monitoring Board for Osiris Therapeutics, and being a founder of Orban Biotech LLC; Dr. Becker reports receiving a grant from Diamyd; Dr. Gitelman reports serving on an advisory board for Genentech; Dr. Golaand reports receiving grants from Diamyd and Tolerx; Dr. Gottlieb reports serving on advisory boards for Genentech, Eli Lilly, Sanofi-Aventis, and Tolerx, and reports receiving grants from Bayhill Therapeutics, Diamyd, Macrogenics, Omni BioTherapeutics, and Tolerx; Dr. Greenbaum reports receiving grants from Bayhill Therapeutics, Diamyd, and Tolerx; Dr. Marks reports serving on an advisory board for Amgen; Dr. Moran reports serving on an advisory board for Pfizer, and receiving grants from Tolerx, Merck, and Osiris Therapeutics; Dr. Raskin reports serving on advisory boards for Amgen, AstraZeneca, MannKind, and Novo-Nordisk, serving on speakers bureaus for Merck and Novo-Nordisk, and receiving grants from Aegera, Andromeda Biotech, Bayhill Therapeutics, Biodel, Boehringer Ingelheim, Calibra, CPEX, Generex, Hoffman-LaRoche, MannKind, Novo-Nordisk, Osiris Therapeutics, and Reata; Dr. Rodriguez reports serving on an advisory board for Marcadia Biotech, serving as a consultant to Eli Lilly, Genentech, Bayer, EMD Serono, and Merck, being on the speakers bureau of Eli Lilly and Novo-Nordisk, and receiving grant support from Macrogenics and Eli Lilly; Dr. Schatz reports serving on advisory boards for Eli Lilly and GlaxoSmithKline, and receiving a grant from Diamyd; Dr. Wherrett reports receiving lecture fees from Eli Lilly and Medtronic; Dr. Wilson reports serving on an advisory boards for DexCom and Genentech, and receiving grants support from Genentech, Diamyd, and Osiris Therapeutics; Dr. Skyler reports serving on boards for Amylin Pharmaceuticals, DexCom, and Sanofi-Aventis, and reports receiving grants from Bayhill Therapeutics, Halozyme, Intuity, and Osiris Therapeutics. No other potential conflict of interest relevant to this article was reported.
Figures
Comment in
-
New hope for immune intervention therapy in type 1 diabetes.Lancet. 2011 Jul 30;378(9789):376-8. doi: 10.1016/S0140-6736(11)60977-X. Epub 2011 Jun 28. Lancet. 2011. PMID: 21719097 No abstract available.
References
-
- von Herrath M, Sanda S, Herold K. Type 1 diabetes as a relapsing-remitting disease? Nat Rev Immunol. 2007;7:988–994. - PubMed
-
- Palmer JP, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. - PubMed
-
- Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial: a randomized, controlled trial. Ann Intern Med. 1998;128:517–523. - PubMed
-
- Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. - PubMed
-
- Herold KC, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–1698. 2002. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 RR024153/RR/NCRR NIH HHS/United States
- U01 DK084565/DK/NIDDK NIH HHS/United States
- HHSN267200800019C/LM/NLM NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK061036/DK/NIDDK NIH HHS/United States
- UL1 RR029890/RR/NCRR NIH HHS/United States
- U01 DK061041/DK/NIDDK NIH HHS/United States
- U01 DK061040/DK/NIDDK NIH HHS/United States
- UC4 DK117009/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
- UL1 RR025744/RR/NCRR NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- UL1 RR031986/RR/NCRR NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01 RR00400/RR/NCRR NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK085505/DK/NIDDK NIH HHS/United States
- U01 DK085463/DK/NIDDK NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- U01 DK061016/DK/NIDDK NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK061055/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical