Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice
- PMID: 21719468
- PMCID: PMC3186294
- DOI: 10.1124/jpet.111.181370
Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice
Abstract
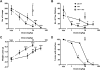
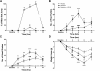
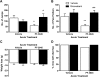
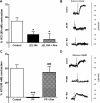
Δ(9)-Tetrahydrocannbinol (THC), the primary active constituent of Cannabis sativa, has long been known to reduce opioid withdrawal symptoms. Although THC produces most of its pharmacological actions through the activation of CB(1) and CB(2) cannabinoid receptors, the role these receptors play in reducing the variety of opioid withdrawal symptoms remains unknown. The endogenous cannabinoids, N-arachidonoylethanolamine (anandamide; AEA) and 2-arachidonylglycerol (2-AG), activate both cannabinoid receptors but are rapidly metabolized by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), respectively. The objective of this study was to test whether increasing AEA or 2-AG, via inhibition of their respective hydrolytic enzymes, reduces naloxone-precipitated morphine withdrawal symptoms in in vivo and in vitro models of opioid dependence. Morphine-dependent mice challenged with naloxone reliably displayed a profound withdrawal syndrome, consisting of jumping, paw tremors, diarrhea, and weight loss. THC and the MAGL inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) dose dependently reduced the intensity of most measures through the activation of CB(1) receptors. JZL184 also attenuated spontaneous withdrawal signs in morphine-dependent mice. The FAAH inhibitor N-(pyridin-3-yl)-4-(3-(5-(trifluoromethyl)pyridin-2-yloxy)benzyl)-piperdine-1-carboxamide (PF-3845) reduced the intensity of naloxone-precipitated jumps and paw flutters through the activation of CB(1) receptors but did not ameliorate incidence of diarrhea or weight loss. In the final series of experiments, we investigated whether JZL184 or PF-3845 would attenuate naloxone-precipitated contractions in morphine-dependent ilea. Both enzyme inhibitors attenuated the intensity of naloxone-induced contractions, although this model does not account mechanistically for the autonomic withdrawal responses (i.e., diarrhea) observed in vivo. These results indicate that endocannabinoid catabolic enzymes are promising targets to treat opioid dependence.
Figures



Similar articles
-
Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice.Neuropsychopharmacology. 2013 May;38(6):1039-49. doi: 10.1038/npp.2012.269. Epub 2013 Jan 3. Neuropsychopharmacology. 2013. PMID: 23303065 Free PMC article.
-
Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain.J Pharmacol Exp Ther. 2009 Sep;330(3):902-10. doi: 10.1124/jpet.109.155465. Epub 2009 Jun 5. J Pharmacol Exp Ther. 2009. PMID: 19502530 Free PMC article.
-
Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice.AAPS J. 2009 Jun;11(2):342-52. doi: 10.1208/s12248-009-9110-7. Epub 2009 May 9. AAPS J. 2009. PMID: 19430909 Free PMC article.
-
Discovery and development of endocannabinoid-hydrolyzing enzyme inhibitors.Curr Top Med Chem. 2010;10(8):828-58. doi: 10.2174/156802610791164238. Curr Top Med Chem. 2010. PMID: 20370710 Review.
-
Targeting fatty acid amide hydrolase (FAAH) to treat pain and inflammation.AAPS J. 2009 Mar;11(1):39-44. doi: 10.1208/s12248-008-9075-y. Epub 2009 Jan 29. AAPS J. 2009. PMID: 19184452 Free PMC article. Review.
Cited by
-
Effect of Pharmacological Modulation of the Endocannabinoid System on Opiate Withdrawal: A Review of the Preclinical Animal Literature.Front Pharmacol. 2016 Jun 28;7:187. doi: 10.3389/fphar.2016.00187. eCollection 2016. Front Pharmacol. 2016. PMID: 27445822 Free PMC article. Review.
-
Differential effects of endocannabinoid catabolic inhibitors on morphine withdrawal in mice.Drug Alcohol Depend. 2015 Jan 1;146:7-16. doi: 10.1016/j.drugalcdep.2014.11.015. Epub 2014 Nov 26. Drug Alcohol Depend. 2015. PMID: 25479915 Free PMC article.
-
Preclinical Studies of Cannabinoid Reward, Treatments for Cannabis Use Disorder, and Addiction-Related Effects of Cannabinoid Exposure.Neuropsychopharmacology. 2018 Jan;43(1):116-141. doi: 10.1038/npp.2017.193. Epub 2017 Aug 28. Neuropsychopharmacology. 2018. PMID: 28845848 Free PMC article. Review.
-
Endocannabinoid signaling regulates the reinforcing and psychostimulant effects of ketamine in mice.Nat Commun. 2020 Nov 24;11(1):5962. doi: 10.1038/s41467-020-19780-z. Nat Commun. 2020. PMID: 33235205 Free PMC article.
-
Chemical probes of endocannabinoid metabolism.Pharmacol Rev. 2013 Mar 19;65(2):849-71. doi: 10.1124/pr.112.006387. Print 2013 Apr. Pharmacol Rev. 2013. PMID: 23512546 Free PMC article. Review.
References
-
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders, 4th ed, American Psychiatric Association, Washington, DC
-
- Basilico L, Parolaro D, Colleoni M, Costa B, Giagnoni G. (1999) Cross-tolerance and convergent dependence between morphine and cannabimimetic agent WIN 55,212-2 in the guinea-pig ileum myenteric plexus. Eur J Pharmacol 376:265–271 - PubMed
-
- Bhargava HN. (1976) Effect of some cannabinoids on naloxone-precipitated abstinence in morphine-dependent mice. Psychopharmacology (Berl) 49:267–270 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DA024009/DA/NIDA NIH HHS/United States
- P50 DA005274/DA/NIDA NIH HHS/United States
- K99 DA030908/DA/NIDA NIH HHS/United States
- T32 DA007027/DA/NIDA NIH HHS/United States
- R01 DA014277/DA/NIDA NIH HHS/United States
- P50-DA005274/DA/NIDA NIH HHS/United States
- P01 DA009789/DA/NIDA NIH HHS/United States
- R01 DA024009/DA/NIDA NIH HHS/United States
- R25 GM090084/GM/NIGMS NIH HHS/United States
- P01-DA017259/DA/NIDA NIH HHS/United States
- R01 DA005488/DA/NIDA NIH HHS/United States
- P01-DA009789/DA/NIDA NIH HHS/United States
- T32-DA007027/DA/NIDA NIH HHS/United States
- 1F3-1DA026279/DA/NIDA NIH HHS/United States
- K99-DA030908/DA/NIDA NIH HHS/United States
- R01-DA014277/DA/NIDA NIH HHS/United States
- R01-DA005488/DA/NIDA NIH HHS/United States
- P01 DA017259/DA/NIDA NIH HHS/United States
- R25 GM089614/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

