Interferon release does not add discriminatory value to smear-negative HIV-tuberculosis algorithms
- PMID: 21719487
- PMCID: PMC3568692
- DOI: 10.1183/09031936.00058911
Interferon release does not add discriminatory value to smear-negative HIV-tuberculosis algorithms
Abstract
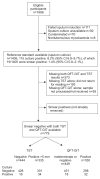
Clinical algorithms for evaluating HIV-infected individuals for tuberculosis (TB) prior to isoniazid preventive therapy (IPT) perform poorly, and interferon-γ release assays (IGRAs) have moderate accuracy for active TB. It is unclear whether, when used as adjunct tests, IGRAs add any clinical discriminatory value for active TB diagnosis in the pre-IPT assessment. 779 sputum smear-negative HIV-infected persons, established on or about to commence combined antiretroviral therapy (ART), were screened for TB prior to IPT. Stepwise multivariable logistic regression was used to develop clinical prediction models. The discriminatory ability was assessed by receiver operator characteristic area under the curve (AUC). QuantiFERON-TB Gold in-tube (QFT-GIT) was evaluated. The prevalence of smear-negative TB by culture was 6.4% (95% CI 4.9-8.4%). Used alone, QFT-GIT and the tuberculin skin test (TST) had comparable performance; the post-test probability of disease based on single negative tests was 3-4%. In a multivariable model, the QFT-GIT test did not improve the ability of a clinical algorithm, which included not taking ART, weight <60 kg, no prior history of TB, any one positive TB symptom/sign (cough ≥ 2 weeks) and CD4+ count <250 cells per mm(3), to discriminate smear-negative culture-positive and -negative TB (72% to 74%; AUC comparison p=0.33). The TST marginally improved the discriminatory ability of the clinical model (to 77%, AUC comparison p=0.04). QFT-GIT does not improve the discriminatory ability of current TB screening clinical algorithms used to evaluate HIV-infected individuals for TB ahead of preventive therapy. Evaluation of new TB diagnostics for clinical relevance should follow a multivariable process that goes beyond test accuracy.
Figures
References
-
- Maartens G, Wilkinson RJ. Tuberculosis. Lancet. 2007;370:2030–2043. - PubMed
-
- World Health Organization . Report of a joint World Health Organization HIV/AIDS and TB department meeting. World health Organization; Geneva: 2008. WHO Three I’s Meeting. Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT), and TB Infection Control (IC) with people living with HIV.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials