STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris
- PMID: 21719692
- PMCID: PMC3160033
- DOI: 10.1105/tpc.111.085340
STENOFOLIA regulates blade outgrowth and leaf vascular patterning in Medicago truncatula and Nicotiana sylvestris
Abstract
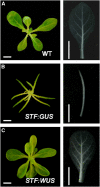
Dicot leaf primordia initiate at the flanks of the shoot apical meristem and extend laterally by cell division and cell expansion to form the flat lamina, but the molecular mechanism of lamina outgrowth remains unclear. Here, we report the identification of STENOFOLIA (STF), a WUSCHEL-like homeobox transcriptional regulator, in Medicago truncatula, which is required for blade outgrowth and leaf vascular patterning. STF belongs to the MAEWEST clade and its inactivation by the transposable element of Nicotiana tabacum cell type1 (Tnt1) retrotransposon insertion leads to abortion of blade expansion in the mediolateral axis and disruption of vein patterning. We also show that the classical lam1 mutant of Nicotiana sylvestris, which is blocked in lamina formation and stem elongation, is caused by deletion of the STF ortholog. STF is expressed at the adaxial-abaxial boundary layer of leaf primordia and governs organization and outgrowth of lamina, conferring morphogenetic competence. STF does not affect formation of lateral leaflets but is critical to their ability to generate a leaf blade. Our data suggest that STF functions by modulating phytohormone homeostasis and crosstalk directly linked to sugar metabolism, highlighting the importance of coordinating metabolic and developmental signals for leaf elaboration.
Figures







Similar articles
-
LOOSE FLOWER, a WUSCHEL-like Homeobox gene, is required for lateral fusion of floral organs in Medicago truncatula.Plant J. 2015 Feb;81(3):480-92. doi: 10.1111/tpj.12743. Epub 2015 Jan 7. Plant J. 2015. PMID: 25492397
-
STENOFOLIA recruits TOPLESS to repress ASYMMETRIC LEAVES2 at the leaf margin and promote leaf blade outgrowth in Medicago truncatula.Plant Cell. 2014 Feb;26(2):650-64. doi: 10.1105/tpc.113.121947. Epub 2014 Feb 28. Plant Cell. 2014. PMID: 24585835 Free PMC article.
-
Control of dicot leaf blade expansion by a WOX gene, STF.Plant Signal Behav. 2011 Nov;6(11):1861-4. doi: 10.4161/psb.6.11.17761. Epub 2011 Nov 1. Plant Signal Behav. 2011. PMID: 22057334 Free PMC article.
-
Leaf adaxial-abaxial polarity specification and lamina outgrowth: evolution and development.Plant Cell Physiol. 2012 Jul;53(7):1180-94. doi: 10.1093/pcp/pcs074. Epub 2012 May 21. Plant Cell Physiol. 2012. PMID: 22619472 Review.
-
Functional Genomics and Genetic Control of Compound Leaf Development in Medicago truncatula: An Overview.Methods Mol Biol. 2018;1822:197-203. doi: 10.1007/978-1-4939-8633-0_14. Methods Mol Biol. 2018. PMID: 30043306 Review.
Cited by
-
ATP-Binding Cassette G Transporters SGE1 and MtABCG13 Control Stigma Exsertion.Plant Physiol. 2020 Sep;184(1):223-235. doi: 10.1104/pp.20.00014. Epub 2020 Jul 20. Plant Physiol. 2020. PMID: 32690757 Free PMC article.
-
Ectopic expression of a wheat WRKY transcription factor gene TaWRKY71-1 results in hyponastic leaves in Arabidopsis thaliana.PLoS One. 2013 May 9;8(5):e63033. doi: 10.1371/journal.pone.0063033. Print 2013. PLoS One. 2013. PMID: 23671653 Free PMC article.
-
Leaf Development in Medicago truncatula.Genes (Basel). 2022 Jul 5;13(7):1203. doi: 10.3390/genes13071203. Genes (Basel). 2022. PMID: 35885986 Free PMC article. Review.
-
Evolutionarily conserved repressive activity of WOX proteins mediates leaf blade outgrowth and floral organ development in plants.Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):366-71. doi: 10.1073/pnas.1215376110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248305 Free PMC article.
-
Overexpression of PvWOX3a in switchgrass promotes stem development and increases plant height.Hortic Res. 2021 Dec 1;8(1):252. doi: 10.1038/s41438-021-00678-w. Hortic Res. 2021. PMID: 34848686 Free PMC article.
References
-
- Barkoulas M., Hay A., Kougioumoutzi E., Tsiantis M. (2008). A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nat. Genet. 40: 1136–1141 - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

