Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis
- PMID: 21719693
- PMCID: PMC3160038
- DOI: 10.1105/tpc.111.087395
Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis
Abstract
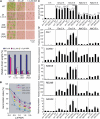
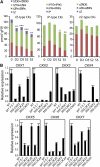
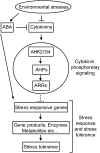
Cytokinins (CKs) regulate plant growth and development via a complex network of CK signaling. Here, we perform functional analyses with CK-deficient plants to provide direct evidence that CKs negatively regulate salt and drought stress signaling. All CK-deficient plants with reduced levels of various CKs exhibited a strong stress-tolerant phenotype that was associated with increased cell membrane integrity and abscisic acid (ABA) hypersensitivity rather than stomatal density and ABA-mediated stomatal closure. Expression of the Arabidopsis thaliana ISOPENTENYL-TRANSFERASE genes involved in the biosynthesis of bioactive CKs and the majority of the Arabidopsis CYTOKININ OXIDASES/DEHYDROGENASES genes was repressed by stress and ABA treatments, leading to a decrease in biologically active CK contents. These results demonstrate a novel mechanism for survival under abiotic stress conditions via the homeostatic regulation of steady state CK levels. Additionally, under normal conditions, although CK deficiency increased the sensitivity of plants to exogenous ABA, it caused a downregulation of key ABA biosynthetic genes, leading to a significant reduction in endogenous ABA levels in CK-deficient plants relative to the wild type. Taken together, this study provides direct evidence that mutual regulation mechanisms exist between the CK and ABA metabolism and signals underlying different processes regulating plant adaptation to stressors as well as plant growth and development.
Figures

Similar articles
-
Arabidopsis type B cytokinin response regulators ARR1, ARR10, and ARR12 negatively regulate plant responses to drought.Proc Natl Acad Sci U S A. 2016 Mar 15;113(11):3090-5. doi: 10.1073/pnas.1600399113. Epub 2016 Feb 16. Proc Natl Acad Sci U S A. 2016. PMID: 26884175 Free PMC article.
-
Roles of four Arabidopsis U-box E3 ubiquitin ligases in negative regulation of abscisic acid-mediated drought stress responses.Plant Physiol. 2012 Sep;160(1):556-68. doi: 10.1104/pp.112.202143. Epub 2012 Jul 24. Plant Physiol. 2012. PMID: 22829319 Free PMC article.
-
AtHAD1, A haloacid dehalogenase-like phosphatase, is involved in repressing the ABA response.Biochem Biophys Res Commun. 2022 Jan 8;587:119-125. doi: 10.1016/j.bbrc.2021.11.095. Epub 2021 Nov 29. Biochem Biophys Res Commun. 2022. PMID: 34871999
-
The Yin-Yang of Cytokinin Homeostasis and Drought Acclimation/Adaptation.Trends Plant Sci. 2016 Jul;21(7):548-550. doi: 10.1016/j.tplants.2016.05.006. Epub 2016 Jun 3. Trends Plant Sci. 2016. PMID: 27270336 Review.
-
Cytokinins: metabolism and function in plant adaptation to environmental stresses.Trends Plant Sci. 2012 Mar;17(3):172-9. doi: 10.1016/j.tplants.2011.12.005. Epub 2012 Jan 9. Trends Plant Sci. 2012. PMID: 22236698 Review.
Cited by
-
Transcript abundance patterns of Populus C-repeat binding factor2 orthologs and genetic association of PsCBF2 allelic variation with physiological and biochemical traits in response to abiotic stress.Planta. 2015 Jul;242(1):295-312. doi: 10.1007/s00425-015-2307-3. Epub 2015 Apr 28. Planta. 2015. PMID: 25916311
-
Transcriptome analyses of a salt-tolerant cytokinin-deficient mutant reveal differential regulation of salt stress response by cytokinin deficiency.PLoS One. 2012;7(2):e32124. doi: 10.1371/journal.pone.0032124. Epub 2012 Feb 15. PLoS One. 2012. PMID: 22355415 Free PMC article.
-
Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L.PLoS One. 2012;7(3):e33210. doi: 10.1371/journal.pone.0033210. Epub 2012 Mar 29. PLoS One. 2012. PMID: 22479371 Free PMC article.
-
Gene Expression Analysis of Microtubers of Potato Solanum tuberosum L. Induced in Cytokinin Containing Medium and Osmotic Stress.Plants (Basel). 2021 Apr 27;10(5):876. doi: 10.3390/plants10050876. Plants (Basel). 2021. PMID: 33925316 Free PMC article.
-
Shared and genetically distinct Zea mays transcriptome responses to ongoing and past low temperature exposure.BMC Genomics. 2018 Oct 20;19(1):761. doi: 10.1186/s12864-018-5134-7. BMC Genomics. 2018. PMID: 30342485 Free PMC article.
References
-
- Achard P., Cheng H., De Grauwe L., Decat J., Schoutteten H., Moritz T., Van Der Straeten D., Peng J., Harberd N.P. (2006). Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 - PubMed
-
- Alvarez S., Marsh E.L., Schroeder S.G., Schachtman D.P. (2008). Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant Cell Environ. 31: 325–340 - PubMed
-
- Argueso C.T., Ferreira F.J., Kieber J.J. (2009). Environmental perception avenues: the interaction of cytokinin and environmental response pathways. Plant Cell Environ. 32: 1147–1160 - PubMed
-
- Bajji M., Lutts S., Kinet J. (2001). Water deficit effects on solute contribution to osmotic adjustment as a function of leaf ageing in three durum wheat (Triticum durum Desf.) cultivars performing differently in arid conditions. Plant Sci. 160: 669–681 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

