Identification of a negative regulatory region for the exchange activity and characterization of T332I mutant of Rho guanine nucleotide exchange factor 10 (ARHGEF10)
- PMID: 21719701
- PMCID: PMC3190991
- DOI: 10.1074/jbc.M111.236810
Identification of a negative regulatory region for the exchange activity and characterization of T332I mutant of Rho guanine nucleotide exchange factor 10 (ARHGEF10)
Abstract
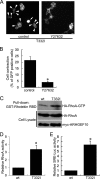
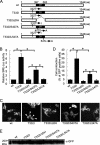
The T332I mutation in Rho guanine nucleotide exchange factor 10 (ARHGEF10) was previously found in persons with slowed nerve conduction velocities and thin myelination of peripheral nerves. However, the molecular and cellular basis of the T332I mutant is not understood. Here, we show that ARHGEF10 has a negative regulatory region in the N terminus, in which residue 332 is located, and the T332I mutant is constitutively active. An N-terminal truncated ARHGEF10 mutant, ARHGEF10 ΔN (lacking amino acids 1-332), induced cell contraction that was inhibited by a Rho kinase inhibitor Y27632 and had higher GEF activity for RhoA than the wild type. The T332I mutant also showed the phenotype similar to the N-terminal truncated mutant. These data suggest that the ARHGEF10 T332I mutation-associated phenotype observed in the peripheral nerves is due to activated GEF activity of the ARHGEF10 T332I mutant.
Figures





Similar articles
-
Regulation of mitotic spindle formation by the RhoA guanine nucleotide exchange factor ARHGEF10.BMC Cell Biol. 2009 Jul 28;10:56. doi: 10.1186/1471-2121-10-56. BMC Cell Biol. 2009. PMID: 19635168 Free PMC article.
-
Slowed conduction and thin myelination of peripheral nerves associated with mutant rho Guanine-nucleotide exchange factor 10.Am J Hum Genet. 2003 Oct;73(4):926-32. doi: 10.1086/378159. Epub 2003 Aug 19. Am J Hum Genet. 2003. PMID: 14508709 Free PMC article.
-
Tumor necrosis factor-induced ArhGEF10 selectively activates RhoB contributing to human microvascular endothelial cell tight junction disruption.FASEB J. 2021 Jun;35(6):e21627. doi: 10.1096/fj.202002783RR. FASEB J. 2021. PMID: 33948992 Free PMC article.
-
Involvement of ARHGEF10, GEF for RhoA, in Rab6/Rab8-mediating membrane traffic.Small GTPases. 2019 May;10(3):169-177. doi: 10.1080/21541248.2017.1302550. Epub 2017 Apr 27. Small GTPases. 2019. PMID: 28448737 Free PMC article. Review.
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
Cited by
-
SCN2A-linked myelination deficits and synaptic plasticity alterations drive auditory processing disorders in ASD.Res Sq [Preprint]. 2024 Aug 28:rs.3.rs-4925935. doi: 10.21203/rs.3.rs-4925935/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Aug 2;16(1):7109. doi: 10.1038/s41467-025-62494-3. PMID: 39257993 Free PMC article. Updated. Preprint.
-
Neuropathy-causing TRPV4 mutations disrupt TRPV4-RhoA interactions and impair neurite extension.Nat Commun. 2021 Mar 4;12(1):1444. doi: 10.1038/s41467-021-21699-y. Nat Commun. 2021. PMID: 33664271 Free PMC article.
-
Genome-Wide Association Study of Campylobacter-Positive Diarrhea Identifies Genes Involved in Toxin Processing and Inflammatory Response.mBio. 2022 Jun 28;13(3):e0055622. doi: 10.1128/mbio.00556-22. Epub 2022 Apr 14. mBio. 2022. PMID: 35420468 Free PMC article.
-
Altered gene expression in blood and sputum in COPD frequent exacerbators in the ECLIPSE cohort.PLoS One. 2014 Sep 29;9(9):e107381. doi: 10.1371/journal.pone.0107381. eCollection 2014. PLoS One. 2014. PMID: 25265030 Free PMC article.
-
8p23.2-pter Microdeletions: Seven New Cases Narrowing the Candidate Region and Review of the Literature.Genes (Basel). 2021 Apr 27;12(5):652. doi: 10.3390/genes12050652. Genes (Basel). 2021. PMID: 33925474 Free PMC article.
References
-
- Etienne-Manneville S., Hall A. (2002) Nature 420, 629–635 - PubMed
-
- Burridge K., Wennerberg K. (2004) Cell 116, 167–179 - PubMed
-
- Ridley A. J., Hall A. (1992) Cell 70, 389–399 - PubMed
-
- Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) Cell 70, 401–410 - PubMed
-
- Nobes C. D., Hall A. (1995) Cell 81, 53–62 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

