Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis
- PMID: 21719782
- PMCID: PMC3137574
- DOI: 10.1681/ASN.2010090970
Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis
Abstract
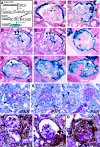
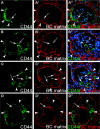
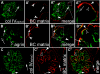
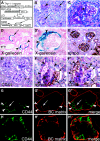
The pathogenesis of the development of sclerotic lesions in focal segmental glomerulosclerosis (FSGS) remains unknown. Here, we selectively tagged podocytes or parietal epithelial cells (PECs) to determine whether PECs contribute to sclerosis. In three distinct models of FSGS (5/6-nephrectomy + DOCA-salt; the murine transgenic chronic Thy1.1 model; or the MWF rat) and in human biopsies, the primary injury to induce FSGS associated with focal activation of PECs and the formation of cellular adhesions to the capillary tuft. From this entry site, activated PECs invaded the affected segment of the glomerular tuft and deposited extracellular matrix. Within the affected segment, podocytes were lost and mesangial sclerosis developed within the endocapillary compartment. In conclusion, these results demonstrate that PECs contribute to the development and progression of the sclerotic lesions that define FSGS, but this pathogenesis may be relevant to all etiologies of glomerulosclerosis.
Copyright © 2011 by the American Society of Nephrology
Figures



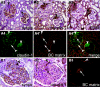
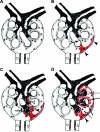
References
-
- Fahr T: Handbuch der speziellen pathologischen Anatomie und Histologie, Vol. VI/1, Berlin, Springer, 1925
-
- Jennette JC, Olson JL, Schwartz MM, Silva FG: Focal Segmental Glomerulosclerosis. In: Heptinstall's Pathology of the Kidney, Philadelphia, Lippincott Williams & Wilkins, 2007, pp 159–175
-
- Yang HC, Fogo AB: ‘Idiopathic’ FSGS: An increasingly obsolete diagnosis? Nephrol Dial Transplant 25: 654–656, 2010 - PubMed
-
- D'Agati V: Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol 23: 117–134, 2003 - PubMed
-
- Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I: Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

