Podocyte injury damages other podocytes
- PMID: 21719786
- PMCID: PMC3137575
- DOI: 10.1681/ASN.2010090963
Podocyte injury damages other podocytes
Abstract
Loss of podocytes promotes glomerulosclerosis, but whether this results from a continued primary insult or a secondary mechanism triggered by the initial loss of podocytes is unknown. We generated chimeric mice in which only a subpopulation of podocytes expressed hCD25, which is the receptor for the immunotoxin LMB2. In addition, genetic labeling of hCD25-negative cells with human placental alkaline phosphatase allowed the study of these two distinct podocyte populations. Administration of LMB2 did not cause podocyte injury in hCD25-negative control mice. In contrast, LMB2 severely damaged or sloughed off the subpopulation of hCD25-positive podocytes within the chimeric glomeruli. Moreover, hCD25-negative podocytes, which were immune to the initial toxin injury, developed injury as early as 4 d after LMB2 injection, evidenced by foot process effacement, upregulation of desmin, and downregulation of nephrin, podocin, and podocalyxin. Furthermore, the magnitude of secondary injury correlated with the magnitude of primary injury, supporting the concept of an amplified cascade of podocyte injury. In conclusion, podocyte damage can propagate injury by triggering secondary damage of "remnant" intact podocytes, even when the primary insult is short-lived. This transmission of podocyte injury may form a vicious cycle leading to accelerated podocyte deterioration and glomerulosclerosis.
Copyright © 2011 by the American Society of Nephrology
Figures

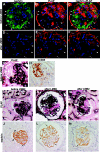
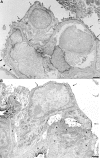
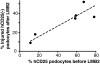
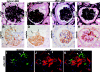

Comment in
-
Podocyte injury can be catching.J Am Soc Nephrol. 2011 Jul;22(7):1181-3. doi: 10.1681/ASN.2011050486. Epub 2011 Jun 16. J Am Soc Nephrol. 2011. PMID: 21680648 No abstract available.
References
-
- LeHir M, Kriz W: New insights into structural patterns encountered in glomerulosclerosis. Curr Opin Nephrol Hypertens 16: 184–191, 2007 - PubMed
-
- D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 - PubMed
-
- Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 - PubMed
-
- Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007 - PubMed
-
- Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

