Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities
- PMID: 21719884
- PMCID: PMC3128214
- DOI: 10.3324/haematol.2010.039669
Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities
Erratum in
- Haematologica. 2011 Aug;96(8):1240. Lindberg, Eva Hellström [corrected to Hellström-Lindberg, Eva]
Abstract
Background: Patients with chromosome 5 abnormalities and high-risk myelodysplastic syndromes or acute myeloid leukemia have a poor outcome. We hypothesized that increasing doses of lenalidomide may benefit this group of patients by inhibiting the tumor clone, as assessed by fluorescence in situ hybridization for del(5q31).
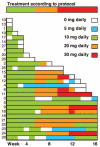
Design and methods: Twenty-eight patients at diagnosis or with relapsed disease and not eligible for standard therapy (16 with acute myeloid leukemia, 12 with intermediate-risk 2 or high-risk myelodysplastic syndrome) were enrolled in this prospective phase II multicenter trial and treated with lenalidomide up to 30 mg daily for 16 weeks. Three patients had isolated del(5q), six had del(5q) plus one additional aberration, 14 had del(5q) and a complex karyotype, four had monosomy 5, and one had del(5q) identified by fluorescence in situ hybridization only.
Results: Major and minor cytogenetic responses, assessed by fluorescence in situ hybridization, were achieved in 5/26 (19%) and 2/26 (8%) patients, respectively, who received one or more dose of lenalidomide, while two patients achieved only a bone marrow response. Nine of all 26 patients (35%) and nine of the ten who completed the 16 weeks of trial responded to treatment. Using the International Working Group criteria for acute myeloid leukemia and myelodysplastic syndrome the overall response rate in treated patients with acute myeloid leukemia was 20% (3/15), while that for patients with myelodysplastic syndrome was 36% (4/11). Seven patients stopped therapy due to progressive disease and nine because of complications, most of which were disease-related. Response rates were similar in patients with isolated del(5q) and in those with additional aberrations. Interestingly, patients with TP53 mutations responded less well than those without mutations (2/13 versus 5/9, respectively; P=0.047). No responses were observed among 11 cases with deleterious TP53 mutations.
Conclusions: Our data support a role for higher doses of lenalidomide in poor prognosis patients with myelodysplastic syndrome and acute myeloid leukemia with deletion 5q. (Clinicaltrials.gov identifier NCT00761449).
Figures
Comment in
-
Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial.Haematologica. 2012 Sep;97(9):e34-5. doi: 10.3324/haematol.2012.067629. Haematologica. 2012. PMID: 22952334 Free PMC article. No abstract available.
References
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. - PMC - PubMed
-
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. - PubMed
-
- Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities amongst 5,876 younger adult patients treated in the UK Medical Research Council trials. Blood. 2010;116(3):354–65. - PubMed
-
- Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

