Phenotypic expression of ADAMTS13 in glomerular endothelial cells
- PMID: 21720563
- PMCID: PMC3123364
- DOI: 10.1371/journal.pone.0021587
Phenotypic expression of ADAMTS13 in glomerular endothelial cells
Abstract
Background: ADAMTS13 is the physiological von Willebrand factor (VWF)-cleaving protease. The aim of this study was to examine ADAMTS13 expression in kidneys from ADAMTS13 wild-type (Adamts13⁺/⁺) and deficient (Adamts13⁻/⁻) mice and to investigate the expression pattern and bioactivity in human glomerular endothelial cells.
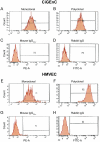
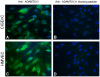
Methodology/principal findings: Immunohistochemistry was performed on kidney sections from ADAMTS13 wild-type and ADAMTS13-deficient mice. Phenotypic differences were examined by ultramorphology. ADAMTS13 expression in human glomerular endothelial cells and dermal microvascular endothelial cells was investigated by real-time PCR, flow cytometry, immunofluorescence and immunoblotting. VWF cleavage was demonstrated by multimer structure analysis and immunoblotting. ADAMTS13 was demonstrated in glomerular endothelial cells in Adamts13⁺/⁺ mice but no staining was visible in tissue from Adamts13⁻/⁻ mice. Thickening of glomerular capillaries with platelet deposition on the vessel wall was detected in Adamts13⁻/⁻ mice. ADAMTS13 mRNA and protein were detected in both human endothelial cells and the protease was secreted. ADAMTS13 activity was demonstrated in glomerular endothelial cells as cleavage of VWF.
Conclusions/significance: Glomerular endothelial cells express and secrete ADAMTS13. The proteolytic activity could have a protective effect preventing deposition of platelets along capillary lumina under the conditions of high shear stress present in glomerular capillaries.
Conflict of interest statement
Figures





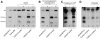

References
-
- Furlan M, Robles R, Lamie B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–4234. - PubMed
-
- Moake JL. von Willebrand factor in thrombotic thrombocytopenic purpura. Blood. 1986;67:1523–1526. - PubMed
-
- Tsai HM, Nagel RL, Hatcher VB, Sussman, II Multimeric composition of endothelial cell-derived von Willebrand factor. Blood. 1989;73:2074–2076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

