Kinase consensus sequences: a breeding ground for crosstalk
- PMID: 21721511
- PMCID: PMC3176959
- DOI: 10.1021/cb200171d
Kinase consensus sequences: a breeding ground for crosstalk
Abstract
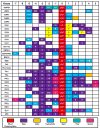
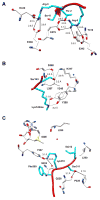
The best characterized examples of crosstalk between two or more different post-translational modifications (PTMs) occur with respect to histones. These examples demonstrate the critical roles that crosstalk plays in regulating cell signaling pathways. Recently, however, non-histone crosstalk has been observed between serine/threonine phosphorylation and the modification of arginine and lysine residues within kinase consensus sequences. Interestingly, many kinase consensus sequences contain critical arginine/lysine residues surrounding the substrate serine/threonine residue. Therefore, we hypothesize that non-histone crosstalk between serine/threonine phosphorylation and arginine/lysine modifications is a global mechanism for the modulation of cellular signaling. In this review, we discuss several recent examples of non-histone kinase consensus sequence crosstalk, as well as provide the biophysical basis for these observations. In addition, we predict likely examples of crosstalk between protein arginine methyltransferase 1 (PRMT1) and Akt and discuss the future implications of these findings.
Figures


References
-
- Suganuma T, Workman JL. Crosstalk among Histone Modifications. Cell. 2008;135:604–607. - PubMed
-
- Fischle W. Talk is cheap--cross-talk in establishment, maintenance, and readout of chromatin modifications. Genes Dev. 2008;22:3375–3382. - PubMed
-
- Schreiber SL, Bernstein BE. Signaling network model of chromatin. Cell. 2002;111:771–778. - PubMed
-
- Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

