Wound repair: toward understanding and integration of single-cell and multicellular wound responses
- PMID: 21721944
- PMCID: PMC4878020
- DOI: 10.1146/annurev-cellbio-092910-154251
Wound repair: toward understanding and integration of single-cell and multicellular wound responses
Abstract
The importance of wound healing to medicine and biology has long been evident, and consequently, wound healing has been the subject of intense investigation for many years. However, several relatively recent developments have added new impetus to wound repair research: the increasing application of model systems; the growing recognition that single cells have a robust, complex, and medically relevant wound healing response; and the emerging recognition that different modes of wound repair bear an uncanny resemblance to other basic biological processes such as morphogenesis and cytokinesis. In this review, each of these developments is described, and their significance for wound healing research is considered. In addition, overlapping mechanisms of single-cell and multicellular wound healing are highlighted, and it is argued that they are more similar than is often recognized. Based on this and other information, a simple model to explain the evolutionary relationships of cytokinesis, single-cell wound repair, multicellular wound repair, and developmental morphogenesis is proposed. Finally, a series of important, but as yet unanswered, questions is posed.
Figures
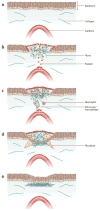




References
-
- Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, et al. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985;20:315–19. - PubMed
-
- Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. - PubMed
-
- Andrews PD, Stark MJ. Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J Cell Sci. 2000;113(Pt. 15):2685–93. - PubMed
-
- Armstrong JR, Ferguson MW. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis domestica. Dev Biol. 1995;169:242–60. - PubMed
-
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–66. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

