Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress
- PMID: 21722091
- PMCID: PMC3149772
- DOI: 10.2174/156720211796558069
Erythropoietin employs cell longevity pathways of SIRT1 to foster endothelial vascular integrity during oxidant stress
Abstract
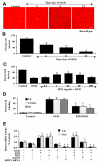
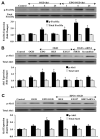
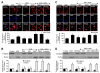
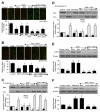
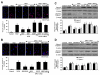
Given the cytoprotective ability of erythropoietin (EPO) in cerebral microvascular endothelial cells (ECs) and the invaluable role of ECs in the central nervous system, it is imperative to elucidate the cellular pathways for EPO to protect ECs against brain injury. Here we illustrate that EPO relies upon the modulation of SIRT1 (silent mating type information regulator 2 homolog 1) in cerebral microvascular ECs to foster cytoprotection during oxygen-glucose deprivation (OGD). SIRT1 activation which results in the inhibition of apoptotic early membrane phosphatidylserine (PS) externalization and subsequent DNA degradation during OGD becomes a necessary component for EPO protection in ECs, since inhibition of SIRT1 activity or diminishing its expression by gene silencing abrogates cell survival supported by EPO during OGD. Furthermore, EPO promotes the subcellular trafficking of SIRT1 to the nucleus which is necessary for EPO to foster vascular protection. EPO through SIRT1 averts apoptosis through activation of protein kinase B (Akt1) and the phosphorylation and cytoplasmic retention of the forkhead transcription factor FoxO3a. SIRT1 through EPO activation also utilizes mitochondrial pathways to prevent mitochondrial depolarization, cytochrome c release, and Bad, caspase 1, and caspase 3 activation. Our work identifies novel pathways for EPO in the vascular system that can govern the activity of SIRT1 to prevent apoptotic injury through Akt1, FoxO3a phosphorylation and trafficking, mitochondrial membrane permeability, Bad activation, and caspase 1 and 3 activities in ECs during oxidant stress.
Figures



References
-
- Bonofiglio R, Lofaro D, Greco R, Senatore M, Papalia T. Proteinuria is a predictor of posttransplant anemia. Transplant Proc. 2011 May;43(4):1063–6. - PubMed
-
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002 Dec 3;106(23):2973–9. - PubMed
-
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003 Mar;23(3):320–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
