The FRP1 F-box gene has different functions in sexuality, pathogenicity and metabolism in three fungal pathogens
- PMID: 21722294
- PMCID: PMC6640539
- DOI: 10.1111/j.1364-3703.2010.00689.x
The FRP1 F-box gene has different functions in sexuality, pathogenicity and metabolism in three fungal pathogens
Abstract
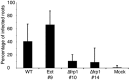
Plant-pathogenic fungi employ a variety of infection strategies; as a result, fungi probably rely on different sets of proteins for successful infection. The F-box protein Frp1, only present in filamentous fungi belonging to the Sordariomycetes, Leotiomycetes and Dothideomycetes, is required for nonsugar carbon catabolism and pathogenicity in the root-infecting fungus Fusarium oxysporum. To assess the role of Frp1 in other plant-pathogenic fungi, FRP1 deletion mutants were generated in Fusarium graminearum and Botrytis cinerea, and their phenotypes were analysed. Deletion of FgFRP1 in F. graminearum led to impaired infection of barley roots, but not of aerial plant parts. Deletion of BcFRP1 in B. cinerea did not show any effect on pathogenicity. Sexual reproduction, however, was impaired in both F. graminearum and B. cinerea FRP1 deletion mutants. The mutants of all three fungi displayed different phenotypes when grown on an array of carbon sources. The F. oxysporum and B. cinerea deletion mutants showed opposite growth phenotypes on sugar and nonsugar carbon sources. Replacement of FoFRP1 in F. oxysporum with the B. cinerea BcFRP1 resulted in the restoration of pathogenicity, but also in a switch from impaired growth on nonsugar carbon sources to impaired growth on sugar carbon sources. This effect could be ascribed in part to the B. cinerea BcFRP1 promoter sequence. In conclusion, the function of the F-box protein Frp1, despite its high sequence conservation, is not conserved between different fungi, leading to differential requirements for pathogenicity and carbon source utilization.
© 2011 The Authors. Molecular Plant Pathology © 2011 BSPP and Blackwell Publishing Ltd.
Figures






References
-
- Cappellini, R.A. and Peterson, J.F. (1965) Macroconidium formation in submerged cultures by a non‐sporulating strain of Gibberella zeae . Mycologia, 57, 962–966.
-
- Choquer, M. , Fournier, E. , Kunz, C. , Levis, C. , Pradier, J.M. and Viaud, A.S.M. (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiol. Lett. 277, 1–10. - PubMed
-
- Cziferszky, A. , Seiboth, B. and Kubicek, C.P. (2003) The Snf1 kinase of the filamentous fungus Hypocrea jecorina phosphorylates regulation‐relevant serine residues in the yeast carbon catabolite repressor Mig1 but not in the filamentous fungal counterpart Cre1. Fungal Genet. Biol. 40, 166–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

