Developmental evolution of flowering plant pollen tube cell walls: callose synthase (CalS) gene expression patterns
- PMID: 21722365
- PMCID: PMC3146827
- DOI: 10.1186/2041-9139-2-14
Developmental evolution of flowering plant pollen tube cell walls: callose synthase (CalS) gene expression patterns
Abstract
Background: A number of innovations underlie the origin of rapid reproductive cycles in angiosperms. A critical early step involved the modification of an ancestrally short and slow-growing pollen tube for faster and longer distance transport of sperm to egg. Associated with this shift are the predominantly callose (1,3-β-glucan) walls and septae (callose plugs) of angiosperm pollen tubes. Callose synthesis is mediated by callose synthase (CalS). Of 12 CalS gene family members in Arabidopsis, only one (CalS5) has been directly linked to pollen tube callose. CalS5 orthologues are present in several monocot and eudicot genomes, but little is known about the evolutionary origin of CalS5 or what its ancestral function may have been.
Results: We investigated expression of CalS in pollen and pollen tubes of selected non-flowering seed plants (gymnosperms) and angiosperms within lineages that diverged below the monocot/eudicot node. First, we determined the nearly full length coding sequence of a CalS5 orthologue from Cabomba caroliniana (CcCalS5) (Nymphaeales). Semi-quantitative RT-PCR demonstrated low CcCalS5 expression within several vegetative tissues, but strong expression in mature pollen. CalS transcripts were detected in pollen tubes of several species within Nymphaeales and Austrobaileyales, and comparative analyses with a phylogenetically diverse group of sequenced genomes indicated homology to CalS5. We also report in silico evidence of a putative CalS5 orthologue from Amborella. Among gymnosperms, CalS5 transcripts were recovered from germinating pollen of Gnetum and Ginkgo, but a novel CalS paralog was instead amplified from germinating pollen of Pinus taeda.
Conclusion: The finding that CalS5 is the predominant callose synthase in pollen tubes of both early-diverging and model system angiosperms is an indicator of the homology of their novel callosic pollen tube walls and callose plugs. The data suggest that CalS5 had transient expression and pollen-specific functions in early seed plants and was then recruited to novel expression patterns and functions within pollen tube walls in an ancestor of extant angiosperms.
Figures
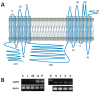

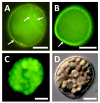


References
-
- Knox RB. In: Cellular Interactions. Linskens HF, Heslop-Harrison J, editor. Berlin, Germany: Springer-Verlag; 1984. Pollen-pistil interactions; pp. 77–92.
-
- Pettitt JM. Detection in primitive gymnosperms of proteins and glycoproteins of possible significance in reproduction. Nature. 1977;266:530. doi: 10.1038/266530a0. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

