Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling
- PMID: 21722728
- PMCID: PMC3205968
- DOI: 10.1016/j.freeradbiomed.2011.06.011
Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling
Abstract
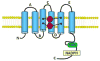
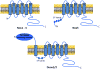
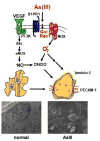
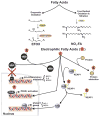
Reactive oxygen species (ROS) are involved in numerous physiological and pathophysiological responses. Increasing evidence implicates ROS as signaling molecules involved in the propagation of cellular pathways. The NADPH oxidase (Nox) family of enzymes is a major source of ROS in the cell and has been related to the progression of many diseases and even environmental toxicity. The complexity of this family's effects on cellular processes stems from the fact that there are seven members, each with unique tissue distribution, cellular localization, and expression. Nox proteins also differ in activation mechanisms and the major ROS detected as their product. To add to this complexity, mounting evidence suggests that other cellular oxidases or their products may be involved in Nox regulation. The overall redox and metabolic status of the cell, specifically the mitochondria, also has implications on ROS signaling. Signaling of such molecules as electrophilic fatty acids has an impact on many redox-sensitive pathologies and thus, as anti-inflammatory molecules, contributes to the complexity of ROS regulation. This review is based on the proceedings of a recent international Oxidase Signaling Symposium at the University of Pittsburgh's Vascular Medicine Institute and Department of Pharmacology and Chemical Biology and encompasses further interaction and discussion among the presenters.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Baldridge CW, Gerard RW. THE EXTRA RESPIRATION OF PHAGOCYTOSIS. AJP - Legacy. 1932;103:235–236.
-
- Iyer GYN, Islam MF, Quastel H. Biochemical Aspects of Phagocytosis. Nature. 1961:535–541.
-
- Bedard K, Krause KH. The NOX family of ROS–generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 BX000513/BX/BLRD VA/United States
- P01 HL095070/HL/NHLBI NIH HHS/United States
- RC1 DK085852/DK/NIDDK NIH HHS/United States
- R01 HL098032/HL/NHLBI NIH HHS/United States
- R01 HL079207/HL/NHLBI NIH HHS/United States
- R01 HL064937/HL/NHLBI NIH HHS/United States
- R01HL098032/HL/NHLBI NIH HHS/United States
- NIH R01HL079207/HL/NHLBI NIH HHS/United States
- R01HL096973/HL/NHLBI NIH HHS/United States
- R01 HL092120/HL/NHLBI NIH HHS/United States
- R37 HL058115/HL/NHLBI NIH HHS/United States
- R01 HL058863/HL/NHLBI NIH HHS/United States
- RC1DK085852/DK/NIDDK NIH HHS/United States
- R37 HL038206/HL/NHLBI NIH HHS/United States
- R01 HL096973/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

