Analysis of individual molecular events of DNA damage response by flow- and image-assisted cytometry
- PMID: 21722802
- PMCID: PMC3132181
- DOI: 10.1016/B978-0-12-385493-3.00006-1
Analysis of individual molecular events of DNA damage response by flow- and image-assisted cytometry
Abstract
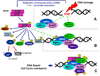
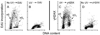
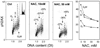
This chapter describes molecular mechanisms of DNA damage response (DDR) and presents flow- and image-assisted cytometric approaches to assess these mechanisms and measure the extent of DDR in individual cells. DNA damage was induced by cell treatment with oxidizing agents, UV light, DNA topoisomerase I or II inhibitors, cisplatin, tobacco smoke, and by exogenous and endogenous oxidants. Chromatin relaxation (decondensation) is an early event of DDR chromatin that involves modification of high mobility group proteins (HMGs) and histone H1 and was detected by cytometry by analysis of the susceptibility of DNA in situ to denaturation using the metachromatic fluorochrome acridine orange. Translocation of the MRN complex consisting of Meiotic Recombination 11 Homolog A (Mre11), Rad50 homolog, and Nijmegen Breakage Syndrome 1 (NMR1) into DNA damage sites was assessed by laser scanning cytometry as the increase in the intensity of maximal pixel as well as integral value of Mre11 immunofluorescence. Examples of cytometric detection of activation of Ataxia telangiectasia mutated (ATM), and Check 2 (Chk2) protein kinases using phospho-specific Abs targeting Ser1981 and Thr68 of these proteins, respectively are also presented. We also discuss approaches to correlate activation of ATM and Chk2 with phosphorylation of p53 on Ser15 and histone H2AX on Ser139 as well as with cell cycle position and DNA replication. The capability of laser scanning cytometry to quantify individual foci of phosphorylated H2AX and/or ATM that provides more dependable assessment of the presence of DNA double-strand breaks is outlined. The new microfluidic Lab-on-a-Chip platforms for interrogation of individual cells offer a novel approach for DDR cytometric analysis.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

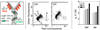





References
-
- Abraham RT, Tibbetts RS. Guiding ATM to broken DNA. Science. 2005;308:510–511. - PubMed
-
- Ahn JY, Li X, Davis HL, Canman CE. Phosphorylation of threonine 68 promotes ologomerization and autophosphorylation of Chk2 protein kinase via the forkhead-associated domain. J. Biol. Chem. 2002;277:19389–19395. - PubMed
-
- Ahn J, Urist M, Prives C. The Chk2 protein kinase. DNA Repair. 2004;3:1039–1047. - PubMed
-
- Albino AP, Huang X, Jorgensen E, Gietl D, Traganos F, Darzynkiewicz Z. Induction of DNA double-strand breaks in A549 and normal human pulmonary epithelial cells by cigarette smoke is mediated by free radicals. Int. J. Oncol. 2006;28:1491–1505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

