Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis
- PMID: 21723126
- PMCID: PMC3143238
- DOI: 10.1016/j.cub.2011.05.048
Structural organization of the actin cytoskeleton at sites of clathrin-mediated endocytosis
Abstract
Background: The dynamic actin cytoskeleton plays an important role in clathrin-mediated endocytosis (CME). However, its exact functions remain uncertain as a result of a lack of high-resolution structural information regarding actin architecture at endocytic sites.
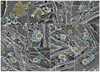
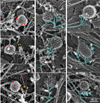
Results: Using platinum replica electron microscopy in combination with electron tomography, we found that actin patches associated with clathrin-coated structures (CCSs) in cultured mouse cells consist of a densely branched actin network, in which actin filament barbed ends are oriented toward the CCS. The shape of the actin network varied from a small lateral patch at the periphery of shallow CCSs, to a collar-like arrangement around partly invaginated CCSs with actin filament barbed ends abutting the CCS neck, to a polarized comet tail in association with highly constricted or fully endocytosed CCSs.
Conclusions: Our data suggest that the primary role of the actin cytoskeleton in CME is to constrict and elongate the bud neck and drive the endocytosed vesicles from the plasma membrane. Moreover, in these processes, barbed ends directly push onto the load, as in a conventional propulsion mechanism. Based on our findings, we propose a model for initiation, evolution, and function of the dendritic actin network at CCSs.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Cell biology: actin keeps endocytosis on a short leash.Curr Biol. 2011 Jul 26;21(14):R552-4. doi: 10.1016/j.cub.2011.06.029. Curr Biol. 2011. PMID: 21783036
References
-
- Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. - PubMed
-
- Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–698. - PubMed
-
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. - PubMed
-
- Merrifield CJ, Perrais D, Zenisek D. Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells. Cell. 2005;121:593–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

